Research that Makes a Difference
Where important, cutting-edge, and novel research happens, you find Pine Research instrumentation products. Pine Research is proud that our electrochemical-based product line has been an integral part of the many research advancements in fields including corrosion inhibition analysis, fuel cell electrocatalyst screening, and battery testing and development.
Corrosion
Corrosion is a naturally occurring electrochemical process that has devastating effects. Corrosion affects material properties such as strength, appearance, and permeability to liquids and gases. Pipelines transporting oil and gas are susceptible to both external and internal corrosion. Understanding the reaction process and contributing variables of corrosion through electrochemical evaluation has played a significant role in product longevity and universal safety. Pine Research Rotating Cylinder Electrodes (RCE) are often employed in routine oilfield operations. The RCE method assesses the rate of pipeline corrosion and therefore enables the evaluation of chemical corrosion inhibitors in specific pipeline systems. The RCE system includes a rotator, which allows technicians to perform flow-based analyses in the laboratory that mimic the same linear velocities and flow conditions in the oilfield.
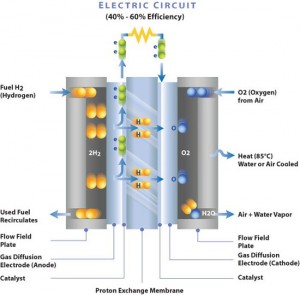
Polymer Electrolyte Membrane (PEM) fuel cell diagram, showing how chemical energy is converted to electrical. “Membrane Electrode Assembly – Electro-Chemical Reaction Diagram” by CFA213FCE – Own work. Licensed under CC BY-SA 3.0 via Commons.
Fuel Cells
For decades, Pine Research instruments have been pivotal in the fuel cell developments industry. One of the most notable uses of our products in fuel cell development is with our Rotating Ring-Disk Electrode systems. One of the most common fuel cells is the analysis of fuel cell electrocatalysts.Our equipment has been in the forefront of the fuel cell research movement. In most types of fuel cells, oxygen (O2) is reduced at the cathode, including polymer electrolyte (PEM), direct methanol (DMFC), alkaline (AFC). and solid oxide (SOFC) fuel cells. The oxygen reduction reaction (ORR) is kinetically slow, which is unfavorable and can lead to diminished fuel cell efficiency. Therefore, catalysts are employed to increase the rate of oxygen reduction reaction. Pine Research electrode rotators and RRDE electrodes are the most widely used tool in use to evaluate the efficiency of ORR catalysts. Researchers in academic and industrial labs depend on us. Advancements in fuel cells have been instrumental in many industries including automobile manufacturers for electric powered vehicles, the US Navy in submarines, and stationary backup power.
Batteries
As electronic technology has enhanced and blossomed over the last couple decades, so has the human need for portable power. Batteries are physical devices that contain one or more voltaic cells, within which chemical energy converts to electrical energy. Such conversion reactions are of great interest to the electrochemical engineer and scientist. These reactions might only occur in one direction (primary, non-rechargeable) or they may occurr in forward and reverse directions (secondary, rechargeable). A great deal of research time and money have been spent of finding the best materials for construction of batteries. Scientists must consider many variables like toxicity, cost and availability of materials, safety, efficiency, and more. Several variables, sometimes loosely referred to as “materials,” must be individually characterized and tested. Often, this is accomplished with electrochemical instrumentation such as potentiostats and battery test stands. In a research setting, fully constructed cells must be charged and discharged many times to determine battery performance and other important variables. In certain circumstances, scientists use Pine Research potentiostats to evaluate many aspects of their novel battery research. From a practical perspective, a large percentage of people carry several batteries on their person at most times of the day – such as in their watches and mobile telephones. Even modern commercial airliners are being outfitted with powerful battery cells, to help minimize electrical load requirements from turbine engine combustion.





