1. Introduction
Corrosion processes can accelerate significantly under extreme environmental conditions such as high temperature, high pressure, and turbulent fluid flow. When troubleshooting a field corrosion problem, a researcher often needs to return to the lab and reproduce the same (or similar) harsh conditions in a controlled setting. While familiar laboratory equipment for temperature control (ovens, water baths) and pressure control (autoclaves) is generally readily available and easy to use, recreating a fluid flow condition generally poses a larger challenge to the researcher.
Laboratory flow loop systems often require complex and expensive plumbing, maintenance, and calibration to reliably and reproducibly move fluid past a metal sample. The need for this type of large scale laboratory equipment can often be avoided by moving the metal sample with respect to the fluid instead.
A convenient instrument1–35 for rapidly moving a metal sample with respect to a fluid is the Rotating Cylinder Electrode (RCE). This apparatus includes an electrode rotator, RCE electrode shaft, and accessories (see above figure) capable of precisely adjusting the rotation rate of a vertically oriented shaft. A special tip capable of holding a cylindrical shaped metal sample is mounted at the lower end of the shaft. The tip is fashioned primarily from chemically inert and electrically insulating materials (such as PTFE, PCTFE, or PEEK), but buried within the tip is a metal shank which provides mechanical stability and also electrical contact with the metal cylinder sample, also called a metal coupon (see figure above). When immersed and rotated in a test solution, the hydrodynamic conditions generated by the RCE, even at low rotation rates, are generally quite turbulent1–5. This makes the RCE an ideal probe for studying corrosion processes12–15 under turbulent conditions, but at low velocity. By adjusting the RCE rotation rate up or down (typically in the range from 200 to 4000 RPM), it is possible to tune the hydrodynamic conditions20–35 adjacent to the metal sample. The ideal goal is to adjust the rotation rate so that the laboratory fluid flow conditions match (or mimic) those found in the field. Once this is accomplished, the corrosion process can be monitored by classic mass loss methods or by electrochemical methods such as Linear Polarization Resistance (LPR)21,22 or Electrochemical Impedance Spectroscopy (EIS).14,24
2. Tuning Turbulent Flow
At very slow rotation rates, the solution near a rotating cylinder flows with a regular and smooth motion called laminar flow. As the rotation rate increases, the solution flow becomes more complex. While the layer of solution in direct contact with the cylinder continues to cling to the surface, the shear stress between this layer and layers further from the cylinder begins to spin off vortices. At this point, the solution flow transitions from laminar to turbulent flow, and as the rotation rate increases, the vortices themselves spawn further vortices.
The transition from laminar to turbulent flow is often characterized using the Reynolds Number to quantify the ratio between inertial forces and viscous forces in a solution. For a rotating cylinder electrode1–3 with outer diameter,
(cm), and radius,
, the Reynold’s Number is
where is the solution density (g/cm3) and µ is the absolute viscosity of the solution (g/cm s). The linear velocity,
(cm/s), at the outer surface of the cylinder is given by
where the rate can either be expressed as angular rotation rate, (rad/s), or as a frequency, F (RPM). In general, for a rotating cylinder, when the Reynolds Number is greater than 200, then the flow is turbulent.
For all but the very slowest rotation rates, the turbulent condition is expected and desired. For a typical Pine Research RCE (15 mm OD, see Figure above), rotation rates between 5 and 4000 RPM correspond to a range of Reynolds Numbers spanning several orders of magnitude (see Table below). The transition from laminar to turbulent flow occurs just above 20 RPM, when the Reynolds Number exceeds 200. It is worth noting that this transition occurs at a relatively small rotation rate, making the RCE an ideal tool for studying turbulent flow at low velocity—precisely the condition frequently found in pipeline infrastructures. Higher turbulent velocities are also easily accessible at higher rotation rates.
3. Mass Transport
The turbulent flow at the RCE can bring material from the solution to the surface of the cylinder, and it can also carry material away from the surface. In the context of a corrosion study, the rate of mass transport to and from the metal surface is often the factor which governs the rate of corrosion. A familiar example would be a corrosion process which is limited by how fast oxygen can be transported from the solution to the metal surface.
Early reports by Eisenberg1,2 provide the most commonly accepted description for RCE mass transport. In particular, the mass transfer coefficient, (cm/s), to a rotating cylinder is given by the following relationship:
where the diffusion coefficient, D (cm2/s) is usually taken as the diffusion coefficient for the molecule or ion undergoing mass transport, and where and
are the dimensionless Sherwood and Reynolds Numbers, respectively. The Schmidt Number,
, is also a dimensionless number.
Combining equations (1) through (3), the overall mass transfer coefficient to an RCE can be expressed in one of three forms,
depending upon whether the rotation rate is expressed in terms of linear surface velocity angular rotation rate
, or rotations per minute (F). Note that the form shown in equation (4) is that which is most often found in the literature.
4. Wall Shear Stress
The turbulent flow at the RCE induces a wall shear stress on the surface of the cylinder. Again, Eisenberg’s original reports1,2 offer a well accepted35 equation for the wall stress, (g/cm s2):
The wall shear stress for a typical Pine Research RCE tip ( = 1.5 cm) over a range of rotation rates is listed in (See Table below)
5. Electrochemical Measurements
When a rotating cylinder is used as the working electrode in a traditional three-electrode cell configuration, the corrosion behavior can be monitored34,35 by measuring the electric current at the cylinder. Electrical connection to the metal cylinder is accomplished by means of a brush contact on the rotating shaft. A potentiostat is employed to impose various potentials on the cylinder electrode while simultaneously measuring the current. The potential signal applied to the cylinder may be a very slow voltage sweep (e.g., Linear Polarization Resistance, LPR),21,22 or it may involve a high frequency sinusoidal signal (i.e., Electrochemical Impedance Spectroscopy, EIS).14,24
Two other electrodes are also required to make an electrochemical measurement, a reference electrode (such as a silver/silver-chloride electrode) and a counter electrode. The counter electrode is often an even larger diameter cylinder, rod, wire loop, or flag placed in the solution so that it surrounds the rotating cylinder. This helps to assure uniform current density at the RCE during the test.
In general, the mass transport limited current density, (A/cm2), observed in an electrochemical experiment is related to the mass transfer coefficient by the following relationship,
where F is Faraday’s Constant (96484.6 C/mol), (A) is the limiting current, and A (cm2) is the area of the electrode. To make full quantitative use of this relationship, both the number of electrons exchanged, z, and the bulk concentration, C of the ion or molecule involved in the electrochemical process must be known.
Combining equations (4) and (6), the mass transport limited current density can be expressed as follows:
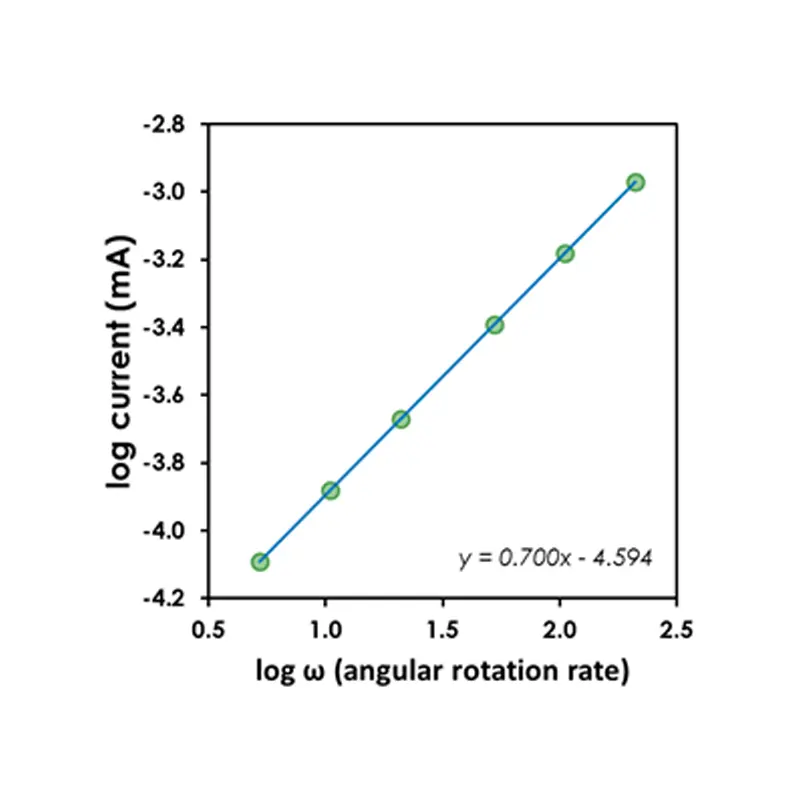
Thus, if a corrosion process is limited by mass transport, it is expected that the limiting current (or limiting current density) will vary linearly with the rotation rate raised to the 0.7 power . Note that this behavior can be verified28 even without explicit knowledge of z and C simply by conducting a set of measurements at several different rotation rates.
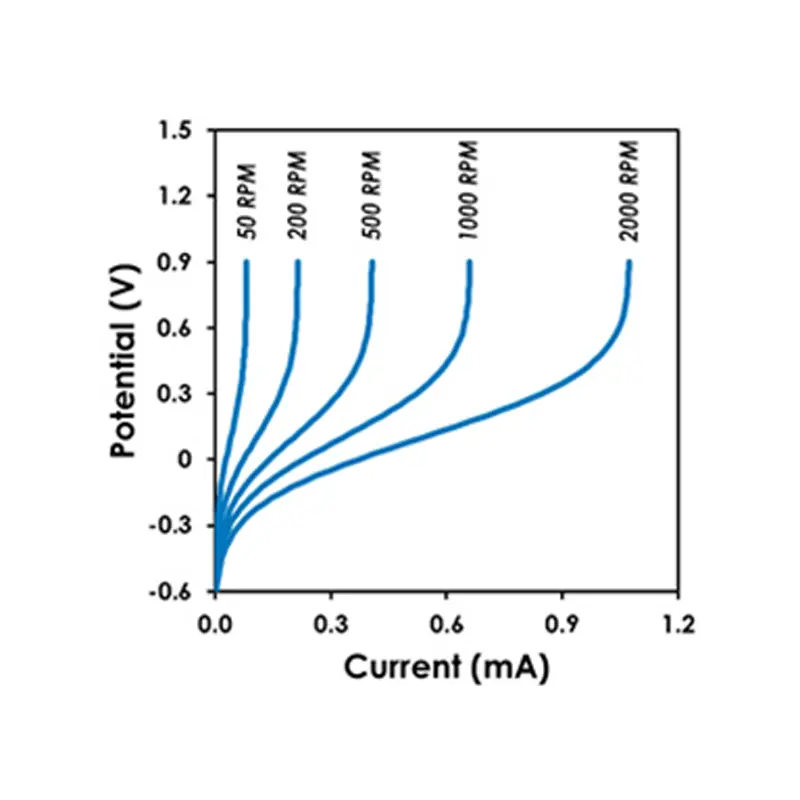
For example, consider a series of LPR scans performed over a range of rotation rates (see figure above). As the rotation rate increases, so does the observed current. A log/log plot of the limiting current (or limiting current density) versus the rotation rate will reveal whether or not the observed current is mass transport limited (see figure above). If the slope of a line drawn through the points on this plot is near 0.7, then this is good evidence that the corrosion process is limited by mass transport.
6. Modeling Pipeline Flow
A critical issue when attempting to use the RCE to match or mimic a field corrosion condition is choosing the proper rotation rate at which to perform electrochemical measurements. Several solutions to this problem have been proposed over the years.20–35 Most involve operating the RCE at a rotation rate where the wall shear stress matches that found in the field, or alternately, at a rate where the mass transport coefficient at the RCE matches that observed in the field.
The discussion here will be limited to the latter case, but at the outset, it is important to note that modeling a field corrosion situation in the laboratory involves some compromise and some assumptions. When an RCE is operated at a rotation rate which produces similar mass transport conditions to those found in the field, it is assumed28 that the corrosion mechanism occurring in the field will be reproduced in the laboratory. However, it is not expected that the actual corrosion rate at the RCE will match that found in the field. There have been specific cases where the RCE failed20 to reproduce the field corrosion condition. Particular attention is required when surface roughness7–10,28 influences mass transport. And lastly, there are practical limitations29 on the range of pipe diameters accessible with the RCE method.
With these caveats in mind, a computational approach outlined in several reports by Silverman21–29 (who, in turn, references reports by Wranglen,30 Holser,31 Chen32 and Nesic)33 is summarized here. Consider turbulent flow through a smooth, straight pipe. If the flow rate through the pipe, (cm/s), is known, then the target surface velocity,
(cm/s), at an RCE which produces a nearly equivalent mass transport condition can be estimated28 as,
where (cm) is the diameter of the pipe. Using this relationship together with equation (2), it is possible to compute a target rotation rate for the RCE.
Equation (11) has too many parameters to plot convenient working curves on a graph. To convey some idea of the type of results produced using this equation, a table below lists the pipe velocity/rotation rate relationships for a typical Pine Research RCE ( = 1.5 cm and A = 3.0 cm2) operating in pure water. As an example, if water is flowing through a smooth 10 in Schedule 40 pipe at 1 ft/s, an RCE should be operated at about 131 RPM to match the conditions in the pipe (see Table below).
7. Corrosion Instrumentation
Pine Research Instrumentation offers specialized tools for the study of mass transport limited corrosion. In addition to our potentiostats for measuring corrosion current and potential, we design and manufacture complete corrosion cell kits. These laboratory corrosion cells have been designed based on input we have received from researchers and practitioners in the corrosion community. The corrosion cell is designed to be sturdy, long lasting, and easily integrated with other Pine products.
The Pine Research classic RCE system was based on the QC012 Series 12 mm OD rotating cylinder electrode (RCE), but an improved system based on the E9 series 15 mm OD rotating cylinder electrode (RCE) is now available. The Pine Research Instrumentation 15 mm OD Rotating Cylinder Electrode System has many features, such as:
- Reliable electrical contact between the shaft and the replaceable cylinder insert is accomplished using a spring-loaded ball plunger which pushes against the inside diameter of the cylinder insert.
- All shaft components are fabricated from a chemically resistant polymer, polyether ether ketone (PEEK), to protect the shaft from corrosive attack during testing. At elevated temperatures (up to 80°C) this polymer has good mechanical stability.
- The 15 mm OD allows for greater wall shear at the cylinder surface for a given rotation rate (as compared to the traditional 12 mm OD design).
- The OpenTop cell features a removable lid for easy cleaning. The chemically resistant Teflon lid has six easily configurable cell ports with standard taper adapters for either 14/20 or 24/25 accessories. With these cell ports, the cell can be configured with a variety of accessories (condenser, pH measurement, thermowell, each sold separately) while still having enough ports for the reference and counter electrode.
- The OpenTop cell features a special recess in the bottom of the cell into which the lower end of the RCE shaft is inserted. This recess assists with aligning the shaft along the axis of the glass cell.
- Achievement of finer temperature control with a jacketed cell design.
- The one liter cell allows for larger solution volumes.
- A dual port purge accessory included with the cell permits the solution to be sparged and/or blanketed with a purge gas. In addition, the gas-purged bearing assembly (through which the rotating shaft enters the cell) has a separate purge port to allow a positive purge pressure to be maintained within the void space of the bearing itself.
The figures above show the components of the 15 mm OD Rotating Cylinder Electrode System. The following additional items are necessary to perform corrosion based measurements:
- An electrochemical workstation to measure corrosion current, like a WaveDriver series electrochemical workstation
- An electrode Rotator to achieve the desired wall shear stress, like the MSR evo Electrode Rotator.
8. Tables
The following data tables have been generated using the equations above.
8.1. 15 mm OD RCE Electrode
8.2. 12 mm OD RCE Electrode
9. References
- Eisenberg, M.; Tobias, C. W.; Wilke, C. R. Ionic Mass Transfer and Concentration Polarization at Rotating Electrodes. J. Electrochem. Soc. 1954, 101, 306.
- Eisenberg, M.; Tobias, C. W.; Wilke, C. R. No Title. Chem. Eng. Progr. Symp. Ser. 1955, 51, 1.
- Gabe, D. R. The Rotating Cylinder Electrode. J. Appl. Electrochem. 1974, 4, 91–108.
- Gabe, D. R.; Robinson, D. J. Mass Transfer in a Rotating Cylinder Cell-I. Laminar Flow. Electrochim. Acta. 1972, 17, 1121–1127.
- Gabe, D. R.; Robinson, D. J. Mass Transfer in a Rotating Cylinder cell—II. Turbulent Flow. Electrochim. Acta. 1972, 17, 1129–1137.
- Gabe, D. R.; Walsh, F. C. The Rotating Cylinder Electrode: A Review of Development. J. Appl. Electrochem. 1983, 13, 3–21.
- Gabe, D. R.; Walsh, F. C. Enhanced Mass Transfer at the Rotating Cylinder Electrode. I. Characterization of a Smooth Cylinder and Roughness Development in Solutions of Constant Concentration. J. Appl. Electrochem. 1984, 14, 555–564.
- Gabe, D. R.; Walsh, F. C. Enhanced Mass Transfer at the Rotating Cylinder Electrode. II. Development of Roughness for Solutions of Decreasing Concentration. J. Appl. Electrochem. 1984, 14, 565–572.
- Gabe, D. R.; Walsh, F. C. Enhanced Mass Transfer at the Rotating Cylinder Electrode: III. Pilot and Production Plant Experience. J. Appl. Electrochem. 1985, 15, 807–824.
- Gabe, D. R.; Makanjuola, P. A. Enhanced Mass Transfer Using Roughened Rotating Cylinder Electrodes in Turbulent Flow. J. Appl. Electrochem. 1987, 17, 370–384.
- Gabe, D. R.; Wilcox, G. D.; Gonzalez-Garcia, J.; Walsh, F. C. The Rotating Cylinder Electrode: Its Continued Development and Application. J. Appl. Electrochem. 1998, 28, 759–780.
- Kear, G.; Barker, B. D.; Stokes, K.; Walsh, F. C. Flow Influenced Electrochemical Corrosion of Nickel Aluminium Bronze – Part II. Anodic Polarisation and Derivation of the Mixed Potential. J. Appl. Electrochem. 2004, 34, 1241–1248.
- Kear, G.; Barker, B. D.; Stokes, K.; Walsh, F. C. Flow Influenced Electrochemical Corrosion of Nickel Aluminium Bronze – Part I. Cathodic Polarisation. J. Appl. Electrochem. 2004, 34, 1235–1240.
- Lu, Q.; Stack, M. M.; Wiseman, C. R. AC Impedance Spectroscopy as a Technique for Investigating Corrosion of Iron in Hot Flowing Bayer Liquors. J. Appl. Electrochem. 2001, 31, 1373–1379.
- Maciel, J. M.; Agostinho, S. M. L. Use of a Rotating Cylinder Electrode in Corrosion Studies of a 90/10 Cu–Ni Alloy in 0.5 Mol L−1 H2SO4 Media. J. Appl. Electrochem. 2000, 30, 981–985.
- Meštrović-Markovinović, A.; Matić, D. Mass Transfer to a Rotating Horizontal Cylinder Electrode with Full and Partial Immersion. J. Appl. Electrochem. 1984, 14, 675–678.
- Grau, J. M.; Bisang, J. M. Mass Transfer Studies at Rotating Cylinder Electrodes of Expanded Metal. J. Appl. Electrochem. 2005, 35, 285–291.
- Eklund, A.; Simonsson, D. Enhanced Mass Transfer to a Rotating Cylinder Electrode with Axial Flow. J. Appl. Electrochem. 1988, 18, 710–714.
- Labraga, L.; Bourabaa, N.; Berkah, T. Wall Shear Stress from a Rotating Cylinder in Cross Flow Using the Electrochemical Technique. Exp. Fluids. 2002, 33, 488–496.
- Efird, K. D.; Wright, E. J.; Boros, J. A.; Hailey, T. G. Correlation of Steel Corrosion in Pipe Flow with Jet Impingement and Rotating Cylinder Tests. Corrosion. 1993, 49, 992–1003.
- Silverman, D. C. Rotating Cylinder Electrode for Velocity Sensitivity Testing. Corrosion. 1984, 40, 220–226.
- Silverman, D. C.; Zerr, M. E. Application of the Rotating Cylinder Electrode—E-Brite 26-1/Concentrated Sulfuric Acid. Corrosion. 1986, 42, 633–640.
- Silverman, D. C. Rotating Cylinder Electrode-Geometry Relationships for Prediction of Velocity-Sensitive Corrosion. Corrosion. 1988, 44, 42–49.
- Silverman, D. C. Corrosion Prediction in Complex Environments Using Electrochemical Impedance Spectroscopy. Electrochim. Acta. 1993, 38, 2075–2078.
- Kalota, D. J.; Silverman, D. C. Behavior of Aspartic Acid as a Corrosion Inhibitor for Steel. Corrosion. 1994, 50, 138–145.
- Silverman, D. C. Technical Note: On Estimating Conditions for Simulating Velocity-Sensitive Corrosion in the Rotating Cylinder Electrode. Corrosion. 1999, 55, 1115–1118.
- Silverman, D. C. Technical Note: Simplified Equation for Simulating Velocity-Sensitive Corrosion in the Rotating Cylinder Electrode at Higher Reynolds Numbers. Corrosion. 2003, 59, 207–211.
- Silverman, D. C. The Rotating Cylinder Electrode for Examining Velocity-Sensitive Corrosion—A Review. Corrosion. 2004, 60, 1003–1023.
- Silverman, D. C. Technical Note: Conditions for Similarity of Mass-Transfer Coefficients and Fluid Shear Stresses between the Rotating Cylinder Electrode and Pipe. Corrosion. 2005, 61, 515–518.
- Levich, V. G. Physicochemical Hydrodynamics; 1st ed.; Prentice-Hall: Englewood Cliffs, NJ, 1962.
- Holser, R. A.; Prentice, G.; Pond, R. B.; Guanti, R. Use of Rotating Cylinder Electrodes to Simulate Turbulent Flow Conditions in Corroding Systems. Corrosion. 1990, 46, 764–769.
- Chen, T. Y.; Moccari, A. A.; Macdonald, D. D. Development of Controlled Hydrodynamic Techniques for Corrosion Testing. Corrosion. 1992, 48, 239–255.
- Nesic, S.; Solvi, G. T.; Skejerve, S. Comparison of Rotating Cylinder and Loop Methods for Testing CO2 Corrosion Inhibitors. Br. Corros. J. 1997, 32, 269–276.
- ASTM G170-06 Standard Guide for Evaluating and Qualifying Oilfield and Refinery Corrosion Inhibitors in the Laboratory. ASTM Int. 2012.
- ASTM G185-06 Standard Practice for Evaluating and Qualifying Oil Field and Refinery Corrosion Inhibitors Using the Rotating Cylinder Electrode. ASTM Int. 2012.