1. Technique Overview
Normal Pulse Voltammetry (NPV) is a derivative technique of Normal Pulse Polarography (NPP). NPP is a technique that was traditionally used with Dropping Mercury Electrodes and Static Mercury Dropping Electrodes. The waveform for the two techniques is the same; however, it is appropriate to use the term “Normal Pulse Voltammetry” when referring to the application of the waveform to nonpolarographic electrodes.
As with many other methods, users can initially sweep positively (towards an anodic limit) or negatively (toward a cathodic limit) initially. Below, typical NPV waveforms are shown for illustration, using only an increasing positive pulse sequence.
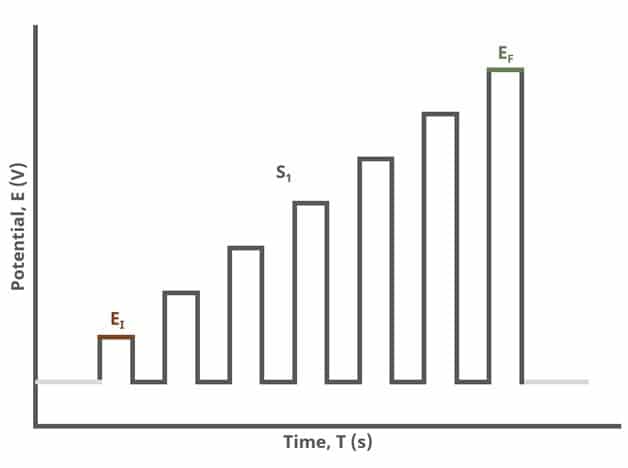
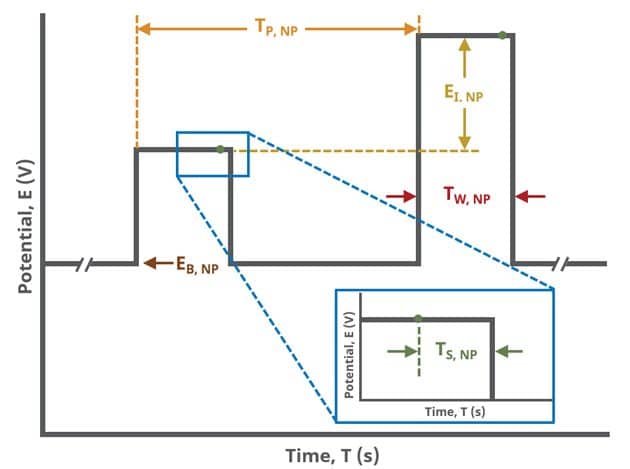
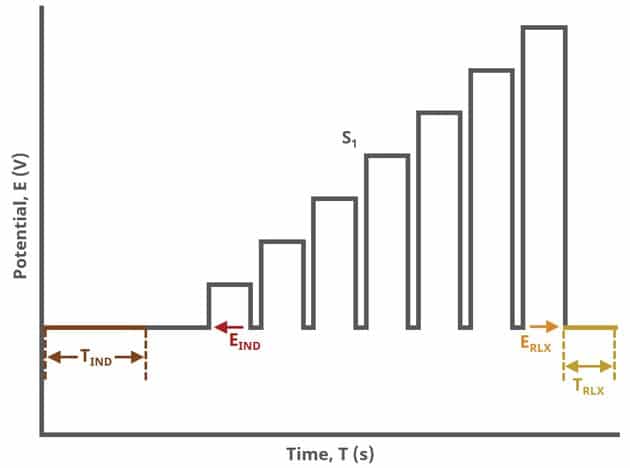
In the simplest case, when Segments (SN) = 1 (see Figure 1), increasing potential pulses step from a common baseline in a linear fashion, from an initial to final potential, sampling current at specified intervals (see Figure 7).
Current sampling occurs at the same time in each pulse of the NPV waveform. For each pulse, the current is sampled as a function of the pulse sequence, , which is determined as
where is the pulse width and
is the sampling width (see Table 1 and Figure 7).
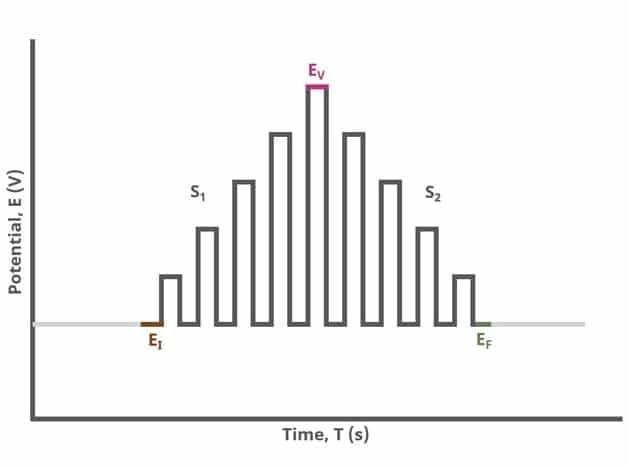
When Segments (SN) = 2 (see Figure 2), increasing potential pulses step from a common baseline in a linear fashion, from an initial potential to vertex potential, at which time the NPV pulses decrease until reaching the final potential. Again, the current is sampled in the same fashion as NPV experiments with other Segment values.
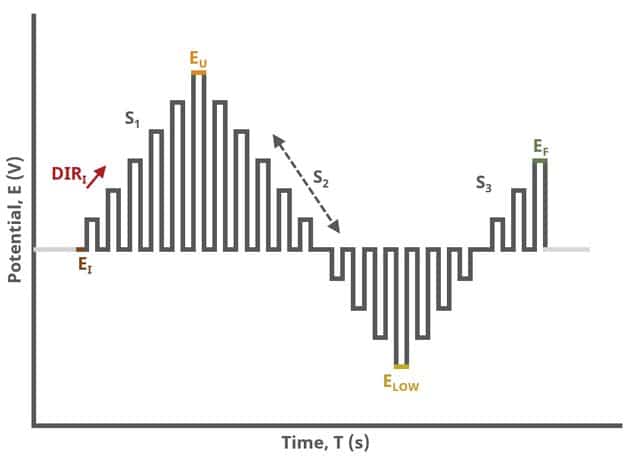
When Segments (SN) = 3 (see Figure 3), increasing potential pulses step from a common baseline in a linear fashion, from an initial potential to upper potential, then from upper potential to lower potential, and finally from lower potential to final potential. Throughout each segment, the NPV pulses increases/decreases until reaching the next significant potential value supplied to AfterMath. Again, the current is sampled in the same fashion as NPV experiments with other Segment values (see Figure 7).
2. Fundamental Equations
Consider the reaction
where is reduced in a one-electron reaction to
with formal potential
. The application of a baseline potential should be sufficiently positive of
such that no faradaic current flows. After a period of time
, typically 100 – 5000 ms, the potential of the working electrode is stepped to a more negative value for a period of time. The total time from the application of the baseline potential through the application of the potential pulse is
.
The potential pulse is incrementally increased with each cycle. As the potential of the working electrode approaches faradaic current flows due to the reduction of
to
. Upon the application of the baseline potential in the next cycle,
is oxidized back to
. When the potential of the working electrode gets sufficiently negative of
,
is reduced to
at a maximum rate and the current levels off to a plateau. The magnitude of this current plateau is given by
where the parameters are as described above. Notice that in the Sample Experiments section, there is a slight anodic current flowing at the beginning of the experiment. The magnitude of this current is given by the equation
where the parameters are as described above. The magnitude of this current is the difference between the NPV and RNPV currents.
Bard and Faulkner1 provide a summary and description of normal pulse voltammetry as do Kissinger and Heinemann2.
3. Experimental Setup in AfterMath
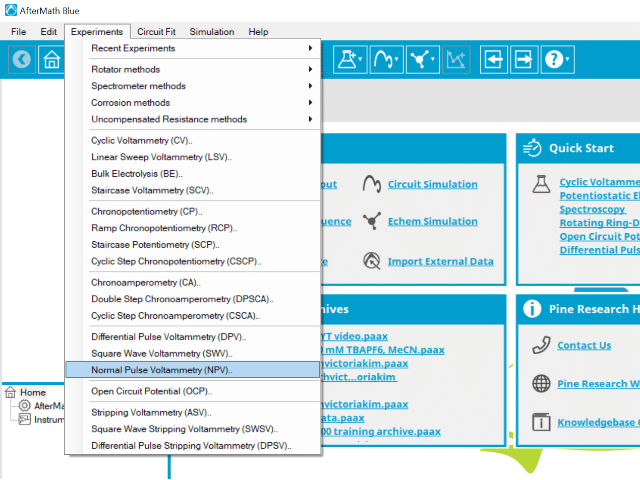
To perform a normal pulse voltammetry experiment in AfterMath, choose Normal Pulse Voltammetry (NPV) from the Experiments menu (see Figure 4).
Doing so creates an entry within the archive, called NPV Parameters. In the right pane of the AfterMath application, several tabs will be shown (see Figure 5).
As with most Aftermath methods, the experiment sequence is
Induction Period → Pulse Sequence → Relaxation Period → Post-Experiment Idle Conditions
Continue reading for detailed information about the fields on each unique tab.
3.1. Basic Tab
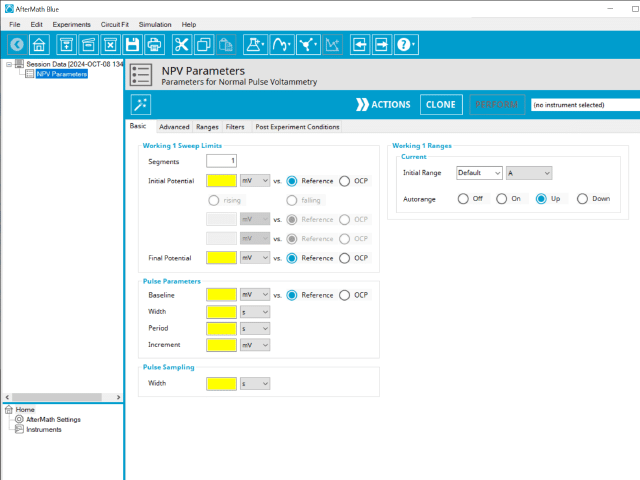
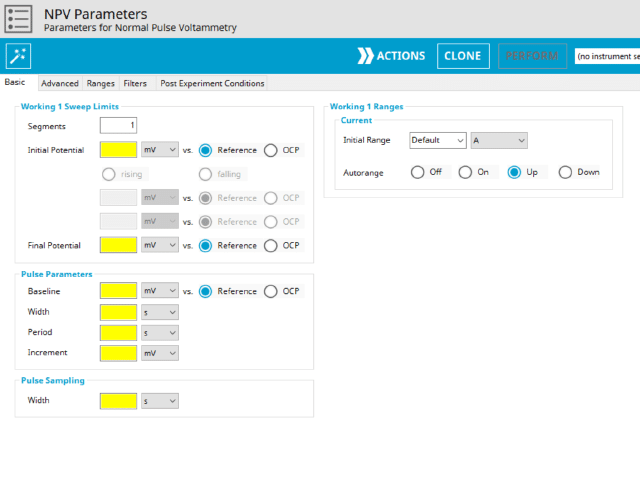
The basic tab contains fields for the fundamental parameters necessary to perform an NPV experiment. AfterMath shades fields with yellow when a required entry is blank and shades fields pink when the entry is invalid (see Figure 6).
During the induction period, a set of initial conditions are applied to the electrochemical cell and the cell equilibrates at these conditions. Data are not collected during the induction period, nor are they shown on the plot during this period. Users will define induction period parameters on the Advanced Tab.
After the induction period, the potential of the working electrode is stepped through a series of increasing pulses from the Initial potential to the Final potential. The potential is incremented with each successive pulse according to the Pulse increment. Current is measured at the time obtained by subtracting the Pulse sampling width window from the Pulse width. In a typical experiment, Segments = 1 and there is a single series of increasing pulses. Some may want to reverse the pulses (move in the opposite direction), accomplished by adjusting the number of segments to be > 1. By adjusting the number of segments, users can create variants of the NPV technique. Cyclic Normal Pulse Voltammetry consists of cycling (through a series of potential pulses also) the potential of the working electrode between an Upper potential and a Lower potential (Segments = 2 or 3). Reverse Normal Pulse Voltammetry is a variant of NPV where you choose to apply a Baseline potential in a region where the Faradaic current is flowing at a maximal rate ( >200 mV from ). Refer to Figures 1, 2, and 3 for examples of such NPV waveforms that vary based on number of segments.
The parameter settings for pulse-type experiments are often not as clear as with something more simple like Cyclic Voltammetry (a sweep method ). Refer to Figure 2 above when understanding each parameter of the NPV pulse. Further, clicking “AutoFill” (“I Feel Lucky” in older versions of AfterMath) provides reasonable starting parameters if you are unsure of typical values used in these experiments. Most often, research journal articles describe the NPV pulse sequence used, which can be replicated using AfterMath.
The experiment concludes with a relaxation period. During the relaxation period, a set of final conditions (specified on the Advanced tab) are applied to the electrochemical cell and the cell equilibrates at these conditions (set on the Advanced Tab). Data are not collected during the induction period, nor are they shown on the plot during this period.
At the end of the relaxation period, the post-experiment idle conditions are applied to the cell and the instrument returns to the idle state.
A plot of the typical experiment sequence, containing labels of the fields on the Basic tab, helps to illustrate the sequence of events in an NPV experiment (see Table 1 and Figure 7).
3.2. Advanced Tab
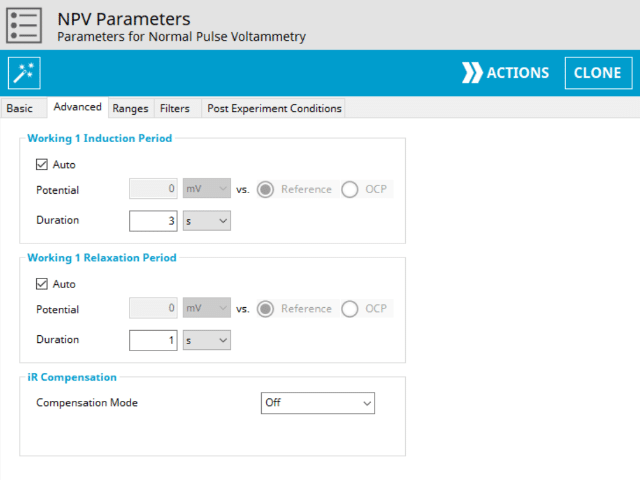
The NPV Advanced tab contains groups for Induction Period, Relaxation Period, and iR Compensation (see Figure 8).
Induction Period is the first step in an NPV experiment if the Duration is >0 s. During the induction period, the specified current is applied to the cell for the specified duration. During this period, data are not collected. The Induction Period is believed to “calm” the cell prior to intentional perturbation. More on Induction Period is found within the knowledgebase.
Relaxation Period is the last step in an NPV experiment if the Duration is >0 s. During the relaxation period, the specified current is applied to the cell for the specified duration. During this period, data are not collected. The Relaxation Period is believed to “calm” the cell after intentional perturbation. More on Relaxation Period is found within the knowledgebase.
Lastly, the iR Compensation group allows users to adjust the cell feedback to accommodate a known resistive drop between working and reference electrodes. Not all potentiostats from Pine Research support iR compensation. The WaveDriver series and WaveNow Wireless series support iR compensation by positive feedback. The WaveNow, WaveNow XV, WaveNano and the CBP bipotentiostat do not support iR compensation.
The general experimental flow for an NPV experiment is provided below (see Figure 9), highlighting the Induction period, NPV Pulse Sequence, and Relaxation period. Following the relaxation period, the post-experiment conditions are applied.
3.3. Range, Filters, and Post Experiment Conditions Tab
In nearly all cases, the groups of fields on the Ranges tab are already present on the Basic tab. The Ranges tab shows an Electrode Range group and depending on the experiment shows either, or both, current and potential ranges and the ability to select an autorange function. The fields on this tab are linked to the same fields on the Basic tab (for most experiments). Changing the values on either the Ranges tab or on the Basic tab changes the other set. In other words, the values selected for these fields will always be the same on the Ranges tab and on the Basic tab. More on ranges is found within the website.
The Filters tab provides access to potentiostat hardware filters, including stability, excitation, current response, and potential response filters. Pine Research recommends that users contact us for help in making changes to hardware filters. Advanced users may have an easier time changing the automatic settings on this tab.
By default, the potentiostat disconnects from the electrochemical cell at the end of an experiment. There are other options available for what these post-experiment conditions can be and are controlled by setting options on the Post Experiment Conditions tab.
4. Sample Experiment
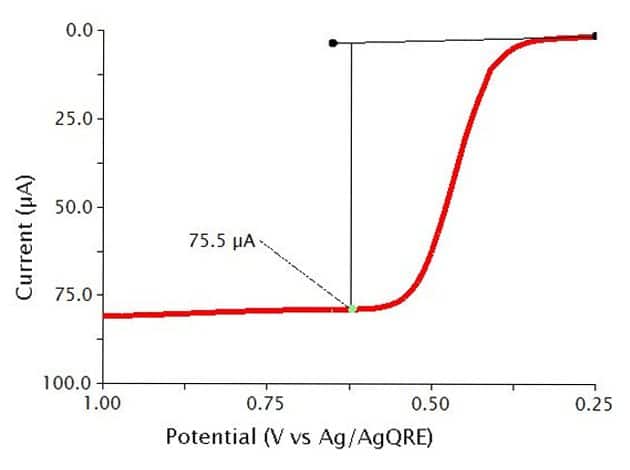
The typical results for NPV of a 0.5 mM solution of Ferrocene in 0.1 M show a sigmoid-shaped curve (see Figure 10). In this experiment, the specific parameters were as follows:
- 2 mm OD Pt WE
- Pt mesh CE
- Baseline Potential = 0.1 V
- Initial Potential = 0.25 V
- Final Potential = 1 V
- Pulse increment = 10 mV
- Pulse period = 100 ms
- Pulse width = 10 ms
- Sample width = 1 ms
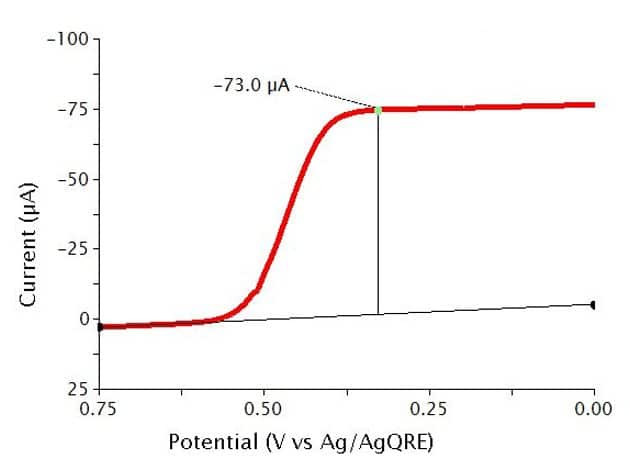
The typical results for Reverse Normal Pulse Voltammetry (RNPV) of a 0.5 mM solution of Ferrocene in 0.1 M show a sigmoid-shaped curve (see Figure 11). In this experiment, the specific parameters were as follows:
- 2 mm OD Pt WE
- Pt mesh CE
- Baseline Potential = 1 V
- Initial Potential = 0.75 V
- Final Potential = 0 V
- Pulse increment = 10 mV
- Pulse period = 100 ms
- Pulse width = 10 ms
- Sample width = 1 ms
5. Example Applications
The first example uses NPV to confirm a diffusion coefficient calculated initially by chronoamperometry (CA). Oyaizu et al.3 produced an organic radical polymer to be used in charge-storage applications. The current in this case is controlled by diffusion of the counter ion through the film.
In another example, Welch et al.4 use NPV to measure a diffusion coefficient. They measured the diffusion coefficients of a free and DNA-bound organo-metallic complex. NPV was superior to CV in this instance due to the low concentration of species in solution.
In this third example, Osteryoung et al. use Reverse Normal Pulse Voltammetry (RNPV). The usefulness of RNPV lies in its ability to examine products from chemical reactions that take place after an electrochemical reaction. Osteryoung et al.5 used RNPV to obtain the backwards rate constant and equilibrium constant for the dimerization of N-methyl-2-carbomethoxypyridinium radical, produced after the electrochemical reduction of the N-methyl-2-carbomethoxypyridinium ion.
6. References
- Bard, A. J.; Faulkner, L. A. Electrochemical Methods: Fundamentals and Applications, 2nd ed. Wiley-Interscience: New York, 2000.
- Kissinger, P.; Heineman, W. R. Laboratory Techniques in Electroanalytical Chemistry, 2nd ed. Marcel Dekker, Inc: New York, 1996.
- Oyaizu, K.; Ando, Y.; Konishi, H.; Nishide, H. Nernstian Adsorbate-like Bulk Layer of Organic Radical Polymers for High-Density Charge Storage Purposes. J. Am. Chem. Soc., 2008, 130(44), 14459-14461.
- Welch, T. W.; Corbett, A. H.; Thorp, H. H. Electrochemical Determination of Nucleic Acid Diffusion Coefficients through Noncovalent Association of a Redox-Active Probe. The Journal of Physical Chemistry, 1995, 99(30), 11757-11763.
- Osteryoung, J.; Talmor, D.; Hermolin, J.; Kirowa-Eisner, E. Reverse pulse voltammetry. Application to second-order following reactions. The Journal of Physical Chemistry, 1981, 85(3), 285-289.