1. General Overview
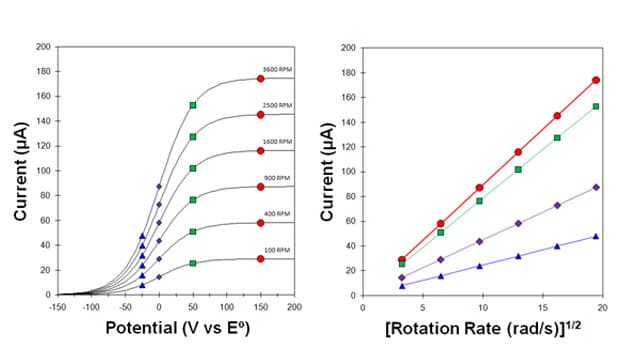
A Levich study is a common experiment performed using a rotating disk electrode in which a series of voltammograms is acquired over a range of different rotation rates.1 For a simple electrochemical system where the rate of the half reaction is governed only by mass transport to the electrode surface, the overall magnitude of the voltammogram should increase with the square root of the rotation rate (see figure below, left).
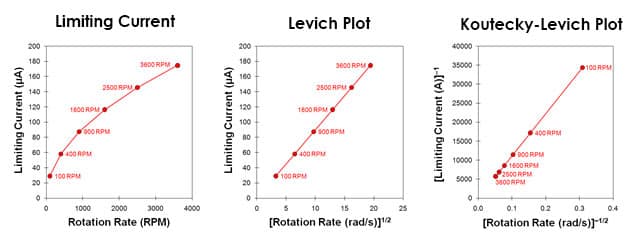
The currents measured during a Levich study are usually plotted against the square root of the rotation rate on a graph called a Levich plot. As predicted by the Levich equation, the limiting current (figure below, red circles) increases linearly with the square root of the rotation rate (with a slope of ) and the line intercepts the vertical axis at zero. It is common to choose a set of rotation rates that are multiples of perfect squares (such as 100, 400, 900, 1600 RPM, etc.) to facilitate construction of this plot.
If the electrochemical half-reaction observed during a Levich study is a simple and reversible half reaction (with no complications due to sluggish kinetics or coupled chemical reactions), then the shapes of the mass-transport controlled voltammograms will be sigmoidal regardless of the rotation rate. This means that the current observed at any given potential along the voltammogram will vary linearly with the square root of the rotation rate (see figure above, right); however, it is important to remember that the Levich equation only applies to the limiting current, not to the currents along the rising portion of the sigmoid.
Because the Levich equation only applies to the limiting current, the results from a Levich experiment are typically presented as a simple plot of the limiting current versus the square root of the rotation rate (see figure above, center). An alternate method of presenting the data from a Levich study is based on a rearrangement of the Levich equation in terms of the reciprocal current.
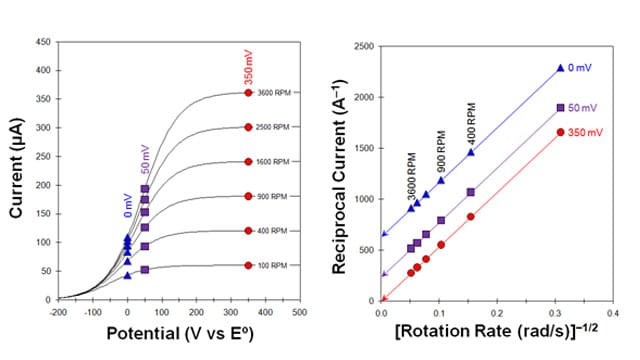
A plot of reciprocal current versus the reciprocal square root of the angular rotation rate (figure below, right) is called a Koutecky-Levich plot.2,3 Again, for a simple and reversible half reaction with no complications, the data fall along a straight line that intercepts the vertical axis at zero. If the line intercepts the vertical axis above zero, however, this is a strong indication that the half-reaction is limited by sluggish kinetics rather than by mass transport.
1.1. References
- Levich, V. G. Physicochemical hydrodynamics, 1st ed. Prentice-Hall: Englewood Cliffs, NJ, 1962.
- Zoski, C. G.; Leddy, J.; Bard, A. J.; Electrochemical Methods: Fundamentals and Applications (Student Solutions Manual), 2nd ed. John Wiley: New York, 2002.
- Treimer, S.; Tang, A.; Johnson, D. C. A Consideration of the Application of Koutecký-Levich Plots in the Diagnoses of Charge-Transfer Mechanisms at Rotated Disk Electrodes. Electroanalysis, 2002, 14(3), 165-171.