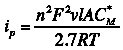This article is part of the AfterMath Data Organizer Electrochemistry Guide[/call_to_action]
[alert icon=”info-circle” color=”blue”]
Species of interest are preconcentrated on an electrode surface or into a Hg electrode (in the form of a Hanging Mercury Dropping Electrode, HMDE or a Mercury Film Electrode, MFE), then essentially “stripped” from the HMDE, MFE, or other electrode surface for qualitative or quantitative purposes. Current is plotted as a function of potential.
Synonyms: Inverse voltammetry
[/alert]Like most of the other electrochemical techniques offered by the AfterMath software, this experiment begins with an induction period, also called a cleaning period for this technique. During this time, a set of initial conditions is applied to the electrochemical cell and the cell is allowed to equilibrate to these conditions. The default initial condition involves holding the working electrode potential at a cleaning potential for a specified period of time.
After the induction period, the potential of the working electrode is stepped to a deposition potential for another specified period of time. Solutions are typically stirred for an HMDE, or a rotating disk electrode (glassy carbon or wax-impregnated graphite) is employed for MFE in order to maintain constant conditions for the deposition process. After the deposition period, stirring is stopped in the case of a HMDE. Next, the potential of the working electrode is linearly swept, or stepped through a series of pulses, from the Initial potential to the Final potential.
After the sequence has finished, the experiment concludes with a relaxation period. The default condition during the relaxation period involves holding the working electrode potential at the final potential for an additional brief period of time (i.e., 1 seconds).
At the end of the relaxation period, the post experiment conditions are applied to the cell and the instrument returns to the idle state.
Current is plotted as a function of the potential applied to the working electrode, resulting in a voltammogram.
The three variants mentioned above, Anodic Stripping Voltammetry (ASV), Differential Pulse Stripping Voltammetry (DPSV), and Square Wave Stripping Voltammetry (SWSV), are three separate experiment types in AfterMath but will all be covered in this wiki. Another variant, called Cathodic Stripping Voltmmetry (CSV) can be employed by using an anodic deposition potential, and is useful in examination of halides. CSV will not be covered in the parameter setup area.
The parameters for these methods are arranged on various tabs on the setup panel. The most commonly used parameters are on the Basic tab, and less commonly used parameters are on the Advanced tab. Each of these two tabs will be discussed separately for ASV, DPSV, and SWSV. Additional tabs for Ranges and Post experiment conditions are common to all of the electrochemical techniques supported by the AfterMath software and will be discussed after the Basic and Advanced tabs sections.
For ASV, you can click on the “I Feel Lucky” button (located at the top of the setup) to fill in all the parameters with typical default values (see Figure 1). Though not typically necessary, you can increase the Duration in the Cleaning period. You may need to change the Potential and Duration in the Deposition period box. Note that you should be several tenths of a volt more negative than the for your species of interest. You will also want to enter a Speed in the Rotator parameters box if you are use an RDE. You may need to change the Initial potential, Final potential, and Sweep rate in the Stripping (sweep) period box to values which are appropriate for the electrochemical system being studied. Finally, you may leave the Electrode Range on Auto or manually choose the expected range of currents. This behavior can also be modified on the Range tab.
Figure 1: Basic setup for ASV.
The waveform that is applied to the electrode is not truly linear, but rather a series of small steps which are generated using the maximum available resolution for the potentiostat's digital-to-analog converter (DAC). Please see the CV wiki for a detailed discussion regarding the waveform applied to the electrode after the deposition step.
The Advanced Tab for this method (see Figure 2) allows you to change the behavior of the potentiostat during the relaxation period. By default, the potential applied to the working electrode during the induction/cleaning and relaxation period will match the initial potential and final potential, respectively, as specified on the Basic Tab. You may override the default behavior of relaxation period if you wish.
Other important parameters on the Advanced tab are found in the Deposition sampling control and Sampling area. The Minimum number of samples in the Deposition sampling control determines how many times the current is measured during the deposition period. The Sampling box contains two parameters, Alpha and Threshold which control when and how samples are acquired during the sweep portion of the experiment.
Figure 2: Advanced parameters for ASV
As mentioned previously, the waveform applied to the electrode is not truly linear (see Figure 3 of CV for a typical waveform). The actual waveform is a staircase of small potential steps. The duration of each small step is called the step period, and the step period is automatically chosen to take full advantage of the resolution of the potentiostat's digital-to-analog converter.
The Alpha parameter controls the exact time within the step period at which the current is sampled. A alpha value of zero means the current is sampled at the start of the step period, immediately after a new potential step is applied. An alpha value of 100 means the current is sampled at the end of the step period, immediately before the next potential step is applied.
Changing alpha will have little effect on the voltammogram for a freely diffusing species in solution; however, variations in alpha can dramatically influence the results for surface bound species as will be the case with ASV, especially when using older potentiostats with low DAC resolution (i.e., 12-bit).
Newer potentiostats (such as the WaveNow and WaveNano portable USB potentiostats) have 16-bit DAC resolution, so voltammograms acquired using these instruments are less influenced by the choice of alpha value. Further details can be found in the literature1 and in a related article about CBP Bipotentiostat Interface Boards.
The Threshold parameter helps you to limit the amount of data retained as the voltammogram is acquired. The threshold parameter controls the interval between samples as the potential is swept from one limit to another. By default, a data point is acquired every time the sweep moves . You can change the threshold from
to a smaller interval (if you want to acquire more data) or to a greater interval (if you want to acquire less data).
Extreme values for the threshold parameter can lead to undesirable results. A smaller threshold value, typically less than or equal to , produces smoother curves and larger data files. If you set the threshold parameter to zero, then the maximum number of data points is acquired (and the size of the resulting data file will be quite large). If you set the parameter to a very large interval, then the number of points acquired will be so small that the voltammogram appears sharp and jagged.
The parameters for DPSV, along with the default values are shown in Figure 3. You will likely need to change the Potential and Duration in the Deposition period box. Note that you should be several tenths of a volt more negative than the for your species of interest. You will also want to enter a Speed in the Rotator parameters box if you are use an RDE. You may need to change the Initial potential and Final potential in the Stripping period baseline box to values which are appropriate for the electrochemical system being studied. The default parameters in the Stripping period pulse box are a good starting point for many DPV experiments. Finally, though you may leave the Electrode Range on Auto, it is recommended that you manually choose the expected range of currents in a pulse experiment.
Figure 3: Basic setup for DPSV.
The waveform that is applied to the electrode in DPV is a sequence of pulses (see Figure 3 of DPV for a typical waveform). The total length of each pulse in the sequence is determined by the Period. The potential of the working electrode is stepped according to the Height for the period of time specified by the Width. The potential of the working electrode is then stepped back but only by the Height minus the Potential increment. The current is sampled at two points in each pulse. The first point is just prior to stepping the potential of the working electrode, and the second point comes at the end of each period just prior to stepping the potential of the working electrode back to begin the next pulse period.
The Advanced Tab for this method (see Figure 4) allows you to change the behavior of the potentiostat during the relaxation period. By default, the potential applied to the working electrode during the induction/cleaning and relaxation periods will match the initial potential and final potential, respectively, as specified on the Basic Tab. You may override the default behavior of relaxation period if you wish.
Figure 4: Basic setup for DPSV.
The typical experimental setup for SWSV is shown in Figure 5. You will likely need to change the Potential and Duration in the Deposition period box. Note that you should be several tenths of a volt more negative than the for your species of interest. You will also want to enter a Speed in the Rotator parameters box if you are use an RDE. You may need to change the Initial potential and Final potential in the Stripping period box to values which are appropriate for the electrochemical system being studied. The default parameters in the Stripping period box relating to the square wave are a good starting point for many SWV experiments. As in DPSV and all other pulse techniques, you may leave the Electrode Range on Auto but it is recommended that you manually choose the expected range of currents in a pulse experiment. This behavior can also be modified on the Range tab.
Figure 5: Basic setup for SWSV.
The Advanced Tab for this method (see Figure 6) allows you to change the behavior of the potentiostat during the relaxation period. By default, the potential applied to the working electrode during the induction/cleaning and relaxation period will match the initial potential and final potential, respectively, as specified on the Basic Tab. You may override the default behavior of relaxation period if you wish.
Other important parameters on the Advanced tab are found in the Deposition sampling control. The Minimum number of samples in the Deposition sampling control determines how many times the current is measured during the deposition period.
Figure 6: Advanced parameters for SWSV
Ranges and Post experiment conditions are common to all of the electrochemical techniques supported by the AfterMath software. Both of these tabs are discussed below.
AfterMath has the ability to automatically select the appropriate ranges for voltage and current during an experiment but as with all pulse techniques, it is best to manually choose the expected range of currents. Please see the separate discussions on autoranging and the Ranges Tab for more information.
After the Relaxation Period, the Post Experiment Conditions are applied to the cell. Typically, the cell is disconnected but you may also specify the conditions applied to the cell. Please see the separate discussion on post experiment conditions for more information.
Below are the typical results for the determination of in solution (
in
with
GC WE, deposition =
@
). Note for quantitation purposes, it is best to build a calibration curve.
Figure 7: ASV of a Lead(II) solution
Figure 8 shows the typical results for the detection of several metal species, with concentrations ranging from to
, in solution (experimental concentrations and conditions are as listed:
,
, and
in
with
,
GC WE, deposition =
@
.
Figure 8: ASV of a Solution of Mixed Metal Species
The following is a brief introduction to to the theory Stripping Voltammetry using a Mercury Film Electrode (MFE). Stripping Voltammetry was traditionally done using an HMDE, which will not be discussed in detail here. For more information, please consult the literature.2 ASV will be discussed first, followed by DPSV, SWSV, and finally CSV. All of the techniques discussed here consist of two steps – 1. Electrodeposition and 2. Stripping. Electrodeposition for the three anodic techniques (ASV, DPSV, and SWSV) is the same and will be discussed first, followed by the stripping analysis for each one individually. CSV will be discussed lastly and independently of the other techniques.
The deposition takes place by applying a potential that is several tenths of a volt more negative than the E0 of the least easily reduced species in solution. Current practice is to add mercuric ion (10 uM to 100 uM) to the solution, so that mercury codeposits with the analyte in the form of a thin film.
At the end of the deposition time in ASV, the analyte is “stripped” from the MFE by sweeping the potential of the electrode linearly to more positive values. Consider the reaction O + e → R, with formal potential E0. All of the material in the MFE is deposited as R. The potential of the electrode is scanned from a value sufficiently negative of the E0 to values sufficiently positive of E0 in order to oxidize R. If the stripping is assumed to be reversible, the Nernst equation holds at the surface and the peak current, ip is given by the the equation
where n and F are as above, v is the scan rate (V/s), l is the film thickness (cm), A is the electrode area (cm2), C is the concentration (mol/cm3), R is the universal gas constant (8.314 J/mol K), and T is the absolute temperature (K). However, the film thickness is not easily obtained so it is easier to use a series of standards of known concentrations to construct a calibration curve for purposes of quantitation.
The theory of DPSV is similar to DPV and has been covered by Copeland et al.3 and Osteryoung and Christie4.
DPV is a technique that is designed to minimize background charging currents. The waveform in DPV is a sequence of pulses, where a baseline potential is held for a specified period of time prior to the application of a potential pulse. Current is sampled, at a point just prior to the application of the potential pulse. The potential is then stepped by a small amount (typically < 100 mV) and current is sampled again at the end of the pulse. The potential of the working electrode is then stepped back by a lesser value than during the forward pulse such that baseline potential of each pulse is incremented throughout the sequence.
Consider a reaction O + e → R, where O is reduced in a one electron step to R. Since deposition was carried out cathodically, the MFE contains only R. At values sufficiently more negative than E0' no faradaic current flows before the potential step (to more positive values). The application of the potential step does not produce an appreciable increase in current. Thus, the differential is very small. At values significantly positive of E0' the baseline potential is oxidizing R at a maximum rate. The application of a small potential step (towards more positive values) is unlikely to increase the rate of oxidation and hence the differential current is again small. Only at potentials around E0' will the differential current be significant. The period during the application of the baseline potential has R being oxidized at some rate. The potential step (to more positive values) increases the rate of oxidation and hence the differential current will be significant.
Interestingly though, DPSV will not offer much in terms of improved sensitivity over ASV since the species of interest is dissolved back into the film with each pulse. The advantage to DPSV is that the charging current is greatly reduced.
The theoretical treatment for SWSV is similar to SWV and has been examined by Knouvaes et al.5 SWSV offers an advantage over both ASV and DPSV in that thinner films actually produce higher sensitivity and peak heights. Maximum peak height is obtained when the film thickness is the same as the diffusion layer thickness.
Consider a reaction O + e → R, where O is reduced in a one electron reaction to R with formal potential, E0. The deposition process causes all O to be reduced to R in the MFE. An initial potential is applied to the electrode that is significantly more negative than E0. No significant faradaic current flows upon the application of forward pulse towards more positive values. Current is sampled at a specified point during the forward pulse.
The reverse pulse consists of stepping the potential of the working electrode to more negative values and the current is sampled near the end of the reverse pulse. The difference current is calculated by subtracting the reverse current from the forward current.
As the potential of the working electrode approaches E0 a faradaic current flows due to oxidation of R. Upon application of the reverse pulse, a faradaic current flows in that effectively reduces the O was was produced during the forward pulse. In other words, the rate of oxidation slows compared to the forward step, hence an cathodic current flows. Once the potential of the working electrode is sufficiently more positive of E0 the current is diffusion-limited in both the forward forward and reverse pulses and the difference current is small.
CSV has been used in the detection of halides, sulfides, selenide, cyanide, cyanoferroate and thiols. No extensive theory shall be presented here regarding this technique, rather you are directed to the numerous reviews (Brainina6, Brainina’s book7, and Vypra8) of stripping voltammetry and references therein regarding CSV. The experimental setup is the opposite of ASV and the underlying electrochemical processes are different. For example, an Ag electrode can be used for the determination of halides, considering the oxidation of silver in the presence of X– produces the insoluble salt, AgX.
The first application is chosen to show the effect of the substrate on the determination of ions in solution. Florence9 compared the stripping curves for an HMDE, pyrolytic graphite, unpolished glassy carbon and polish glassy carbon electrodes. The graphite and glassy carbon electrodes were rotated in the solutions that also contained Hg2+ in order to produce a thin film. Depositions times were 30 minutes for the HMDE and 5 minutes for the other electrodes. In the final side-by-side comparison, the three carbon-based electrodes showed superior selectivity with the polished glassy carbon electrode also showing the best signal-to-noise ratio of the three.
The second application utilizes DPSV for determination of Hg in seawater. Gustavvson10 deposited Hg on to a rotating Au disc electrode. By removing interfering organic material through decomposition with UV light prior to analysis, the researcher was able to detect Hg at concentrations as low as 10 pM.
In the third example, Wang et al.11 used bismuth-coated carbon electrodes for the detection of cadmium, lead, thallium, and zinc. Bi is an attractive alternative to Hg from an environmental standpoint but also from a selectivity standpoint since the formal potentials in Bi are slightly different than in Hg. The researchers used short depositions times (2-10 minutes) to create the thin films and square wave voltammetry for the determination down to the ppb level.
The fourth application is an example of CSV. Piche and Kubiak12 used CSV to detect arsenic at concentrations as low as 0.06 nM. The researchers speculate that copper and sodium diethyldithiocarbamate aid to form an amalgam with Ar(III) on the surface of the electrode in as little as 50 s. Upon sweeping cathodically, Ar(III) is reduced to ArH3. One of the advantages with this method is the ability to discern Ar(III) from Ar(V) making it superior to methods such as ICP-MS and Atomic Absortpion Spectroscopy.
- He, P. Anal. Chem., 1995, 67, 986-992.
- Faulkner, L. R.; Bard, A. J. Bulk Electrolysis Methods. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New Jersey, 2000; 417-470.
- Copeland, T. R.; Christie, J. H.; Osteryoung, R. A.; Skogerboe, R. K. Anal. Chem. 1973, 45, 2171-2174.
- Osteryoung, R. A.; Christie, J. H. Anal. Chem. 1974, 46, 351-355.
- Knouvaes, S. P.; O'Dea, J. J.; Chandresekhar, P.; Osteryoung, J. Anal. Chem. 1986, 58, 3199-3202.
- Brainina, K. Z. Talanta 1971, 18, 513.
- Brainina, K. Z., translated to English by P. Sheinitz, Stripping Voitammetry in Chemical Analysis, Wiley: New York, 1974.
- Vypra, F.; Stulyk, K.; Juakova, E., translated to English by J. Tyson, Electrochemical Stripping Analysis, Wiley: New York, 1976.
- Florence, T. M. J. Electroanal. Chem. 1970, 27, 273-281.
- Gustavvson, I. J. Electroanal. Chem. 1986, 214, 31-36.
- Wang, J.; Lu, J.; Hocevar, S. B.; Farias, P. A. M.; Ogorevc, B. Anal. Chem. 2000, 72, 3218-3222.
- Piche, R.; Kubiak, W. W. J. Electroanal. Chem. 2007, 599, 59-64.
Links: Electrochemist's Guide, AfterMath User's Guide, AfterMath Main Support Page
Related Techniques: Differential Pulse Voltammetry (DPV), Square Wave Voltammetry (SWV)