Reference Electrode, Silver/Silver Chloride, Single Junction
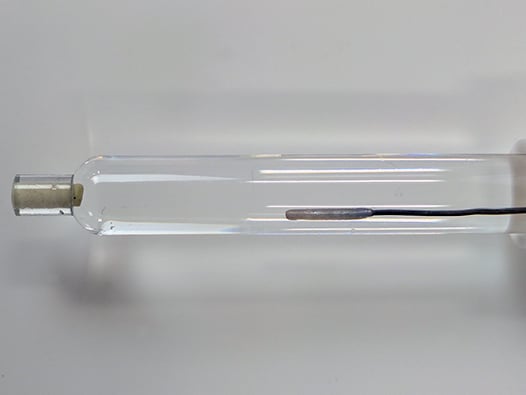
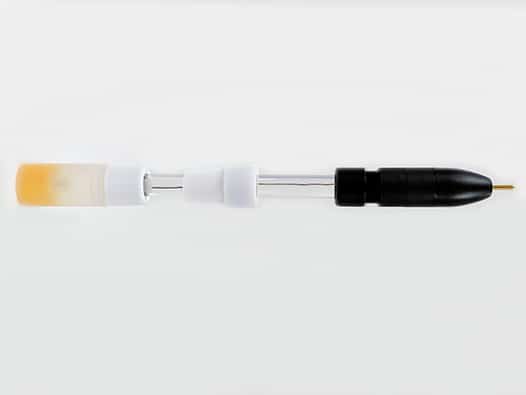
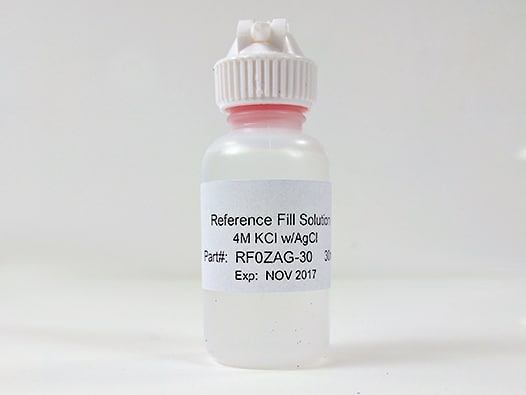
The standard-sized, single junction, silver/silver chloride (Ag/AgCl) reference electrode is filled with 4M KCl with AgCl solution. The refrerence electrode body is 9.5 mm OD, therefore ideally used with Standard volume electrochemical cells. The reference electrode is designed to be pass through a 14/20 ground glass joint and held in place by the included PTFE adapter, which can be adjusted to control the immersion depth of the reference electrode into the cell. Pine Research recommends proper storage and use of reference electrodes. A variety of useful tools for storage and care are shown below.
This product consists of separately available parts. View the parts list in the tab below.
Customers must be logged into their account to view prices. Not all regions provide pricing online. If you do not see prices, you can obtain them from the designated sales channel in your region.
The standard-sized, single junction, silver/silver chloride (Ag/AgCl) reference electrode is filled with 4M KCl with AgCl solution. The refrerence electrode body is 9.5 mm OD, therefore ideally used with Standard volume electrochemical cells. The reference electrode is designed to be pass through a 14/20 ground glass joint and held in place by the included PTFE adapter, which can be adjusted to control the immersion depth of the reference electrode into the cell. Pine Research recommends proper storage and use of reference electrodes. A variety of useful tools for storage and care are shown below.