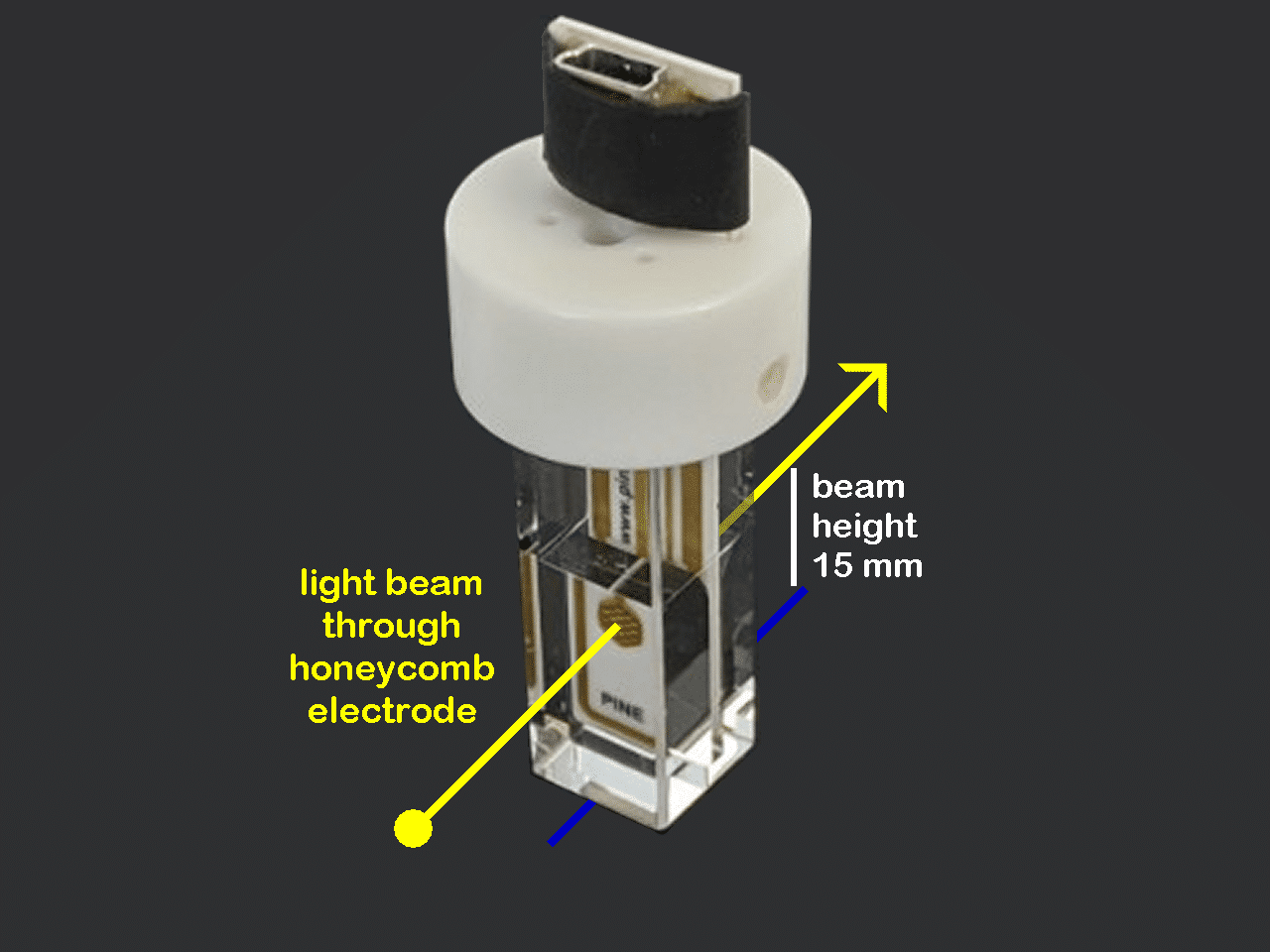
Our unique UV/Vis spectroelectrochemical cell features a patterned “honeycomb” electrode which mounts easily inside a thin-layer quartz cuvette. A special cuvette cap securely holds the honeycomb electrode card and a separate reference electrode in the proper position within the cuvette.
Complete SpectroelectrochemistryCell Kit: The Honeycomb Spectroelectrochemical Cell Kit (AKSTCKIT3) includes 1 quartz cuvette, 1 cell cap, 1 platinum honeycomb electrode, 2 gold honeycomb electrodes, the Universal Specialty Cell Connection Kit, and a LowProfile Ag/AgCl reference electrode. Our honeycomb electrode chip contains an onboard working, counter and reference electrode. If an alternative reference electrode is needed, we offer a cell cable with a separate reference breakout lead with each Honeycomb cell, for use with any potentiostat.
- Jeon, H.; Jo, H.; Seo, S.; Lee, S.; Yoon, S.; Han, D. In-situ spectroelectrochemical analysis: Irreversible deformation of cesium lead bromide Perovskite Quantum Dots in SiOx matrices. Sensors and Actuators Reports 2024, 8, 100208.
- Sahil, S.T.; McCardle, K.M.; Le Magueres, P.; Panetier, J.A.; Jurss, J.W. Investigations of a Copper(II) Bipyridyl-N-Heterocyclic Carbene Macrocycle for CO2 Reduction: Apparent Formation of an Imidazolium Carboxylate Intermediate Leading to Demetalation. ACS Omega 2024, 9, 34555-34566.
- Milunovic, M.N.M.; Ohui, K.; Besleaga, I.; Petrasheuskaya, T.V.; Dömötör, O.; Enyedy, É.A.; Darvasiova, D.; Rapta, P.; Barbieriková, Z.; Vegh, D.; Tóth, S.; Tóth, J.; Kucsma, N.; Szakács, G.; Popović-Bijelić, A.; Zafar, A.; Reynisson, J.; Shutalev, A.D.; Bai, R.; Hamel, E.; Arion, V.B. Copper(II) Complexes with Isomeric Morpholine-Substituted 2-Formylpyridine Thiosemicarbazone Hybrids as Potential Anticancer Drugs Inhibiting Both Ribonucleotide Reductase and Tubulin Polymerization: The Morpholine Position Matters. J. Med. Chem. 2024, 67, 9069-9090.
- Kovács, H.; Jakusch, T.; May, N.V.; Tóth, S.; Szakács, G.; Enyedy, É.A. Complex formation of ML324, the histone demethylase inhibitor, with essential metal ions: Relationship between solution chemistry and anticancer activity. J. Inorg. Biochem. 2024, 255, 112540.
- Gemünde, A.; Gail, J.; Holtmann, D. Redox mediator interaction with Cupriavidus necator – spectroelectrochemical online analysis. Electrochem. Commun. 2024, 162, 107705.
- Portela, P.C.; Shipps, C.C.; Shen, C.; Srikanth, V.; Salgueiro, C.A.; Malvankar, N.S. Widespread extracellular electron transfer pathways for charging microbial cytochrome OmcS nanowires via periplasmic cytochromes PpcABCDE. Nat. Commun. 2024, 15, 2434.
- Zhang, D.; E. Rosch, L.; R. Crawley, M.; R. Cook, T. Post-synthetic modification of bis-iron( iii )-μ-oxo-porphyrin prisms to enhance oxygen reduction electrocatalysis. Inorg. Chem. Front. 2024, 11, 5557-5565.
- Lee, C.; Kim, K.; Shin, Y.; Han, D.; Yoon, S.J. In Situ Spectroelectrochemical Investigation of Perovskite Quantum Dots for Tracking Their Transformation. Frontiers in Energy Research 2021, 8, -.
- Carpenter, J.M.; Zhong, F.; Ragusa, M.J.; Louro, R.O.; Hogan, D.A.; Pletneva, E.V. Structure and redox properties of the diheme electron carrier cytochrome c4 from Pseudomonas aeruginosa. J. Inorg. Biochem. 2020, 203, 110889.
- Balaraman, L.; Emhoff, K.A.; Salem, A.M.; Hanna, J.; Alsabony, M.N.; Bayachou, M.; Mundell, J.J.; Christopher Boyd, W. Electrochemical studies of cobalt(II) diphenylazodioxide complexes. Inorg. Chim. Acta 2020, 501, 119277.
- Darvasiová, D.; Šoral, M.; Puškárová, I.; Dvoranová, D.; Vénosová, B.; Bučinský, L.; Zalibera, M.; Dujnič, V.; Dobrov, A.; Schwalbe, M.; Arion, V.B.; Rapta, P. Spectroelectrochemical, photochemical and theoretical study of octaazamacrocyclic nickel(II) complexes exhibiting unusual solvent-dependent deprotonation of methylene group. Electrochim. Acta 2019, 326, 135006.
- Zheng, W.; Tsang, C.; So, L.Y.; Liu, M.; Leung, Y.; Lee, L.Y.S. Highly efficient stepwise electrochemical degradation of antibiotics in water by in situ formed Cu(OH)2 nanowires. Applied Catalysis B: Environmental 2019, 256, 117824.
- Dobrov, A.; Darvasiová, D.; Zalibera, M.; Bučinský, L.; Puškárová, I.; Rapta, P.; Shova, S.; Dumitrescu, D.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; Arion, V.B. Nickel(II) Complexes with Redox Noninnocent Octaazamacrocycles as Catalysts in Oxidation Reactions. Inorg. Chem. 2019, 58, 11133-11145.
- Ohui, K.; Babak, M.V.; Darvasiova, D.; Roller, A.; Vegh, D.; Rapta, P.; Guan, G.R.S.; Ou, Y.H.; Pastorin, G.; Arion, V.B. Redox-Active Organoruthenium(II)– and Organoosmium(II)–Copper(II) Complexes, with an Amidrazone–Morpholine Hybrid and [CuICl2]− as Counteranion and Their Antiproliferative Activity. Organometallics 2019, 38, 2307-2318.
- Ohui, K.; Afanasenko, E.; Bacher, F.; Ting, R.L.X.; Zafar, A.; Blanco-Cabra, N.; Torrents, E.; Dömötör, O.; May, N.V.; Darvasiova, D.; Enyedy, É.A.; Popović-Bijelić, A.; Reynisson, J.; Rapta, P.; Babak, M.V.; Pastorin, G.; Arion, V.B. New Water-Soluble Copper(II) Complexes with Morpholine–Thiosemicarbazone Hybrids: Insights into the Anticancer and Antibacterial Mode of Action. J. Med. Chem. 2019, 62, 512-530.
- Shova, S.; Vlad, A.; Cazacu, M.; Krzystek, J.; Ozarowski, A.; Malček, M.; Bucinsky, L.; Rapta, P.; Cano, J.; Telser, J.; B. Arion, V. Dinuclear manganese(III) complexes with bioinspired coordination and variable linkers showing weak exchange effects: a synthetic, structural, spectroscopic and computation study. Dalton Trans. 2019.
- Brady, M. Fundamental Insights into Dye-Sensitized Interfaces for Solar Fuels Production. Ph.D. Dissertation, University of North Carolina at Chapel Hill, 2019.
- Schorsch, M.; Kramer, M.; Goss, T.; Eisenhut, M.; Robinson, N.; Osman, D.; Wilde, A.; Sadaf, S.; Brückler, H.; Walder, L.; Scheibe, R.; Hase, T.; Hanke, G.T. A unique ferredoxin acts as a player in the low-iron response of photosynthetic organisms. Proceedings of the National Academy of Sciences 2018, 115, E12111-E12120.
- Kellett, C.W.; Swords, W.B.; Turlington, M.D.; Meyer, G.J.; Berlinguette, C.P. Resolving orbital pathways for intermolecular electron transfer. Nat. Commun. 2018, 9, 4916.
- Orlowska, E.; Babak, M.V.; Dömötör, O.; Enyedy, E.A.; Rapta, P.; Zalibera, M.; Bučinský, L.; Malček, M.; Govind, C.; Karunakaran, V.; Farid, Y.C.S.; McDonnell, T.E.; Luneau, D.; Schaniel, D.; Ang, W.H.; Arion, V.B. NO Releasing and Anticancer Properties of Octahedral Ruthenium–Nitrosyl Complexes with Equatorial 1H-Indazole Ligands. Inorg. Chem. 2018, 57, 10702-10717.
- Piechota, E.J.; Troian-Gautier, L.; Sampaio, R.N.; Brennaman, M.K.; Hu, K.; Berlinguette, C.P.; Meyer, G.J. Optical Intramolecular Electron Transfer in Opposite Directions through the Same Bridge That Follows Different Pathways. J. Am. Chem. Soc. 2018, 140, 7176-7186.
- Shova, S.; Vlad, A.; Cazacu, M.; Krzystek, J.; Bucinsky, L.; Breza, M.; Darvasiová, D.; Rapta, P.; Cano, J.; Telser, J.; B. Arion, V. A five-coordinate manganese(III) complex of a salen type ligand with a positive axial anisotropy parameter D. Dalton Trans. 2017, 46, 11817-11829.
- E. Büchel, G.; Kossatz, S.; Sadique, A.; Rapta, P.; Zalibera, M.; Bucinsky, L.; Komorovsky, S.; Telser, J.; Eppinger, J.; Reiner, T.; B. Arion, V. cis -Tetrachlorido-bis(indazole)osmium(IV) and its osmium(III) analogues: paving the way towards the cis -isomer of the ruthenium anticancer drugs KP1019 and/or NKP1339. Dalton Trans. 2017, 46, 11925-11941.
- Barr, T.J.; Morris, A.J.; Taheri, A.; Meyer, G.J. Charge Rectification at Molecular Nanocrystalline TiO2 Interfaces: Overlap Optimization To Promote Vectorial Electron Transfer. The Journal of Physical Chemistry C 2016, 120, 27173-27181.
- Adams, R.E.; Schmehl, R.H. Micellar Effects on Photoinduced Electron Transfer in Aqueous Solutions Revisited: Dramatic Enhancement of Cage Escape Yields in Surfactant Ru(II) Diimine Complex/[Ru(NH3)6]2+ Systems. Langmuir 2016, 32, 8598–8607.
- Liang, Z.; Kang, M.; Payne, G.F.; Wang, X.; Sun, R. Probing Energy and Electron Transfer Mechanisms in Fluorescence Quenching of Biomass Carbon Quantum Dots. ACS Appl. Mater. Interfaces 2016, 8, 17478-17488.
- Salpage, S.R.; Paul, A.; Som, B.; Banerjee, T.; Hanson, K.; Smith, M.D.; Vannucci, A.K.; Shimizu, L.S. Structural, electrochemical and photophysical properties of an exocyclic di-ruthenium complex and its application as a photosensitizer. Dalton Trans. 2016, 45, 9601-9607.
- Al-Yasari, A. Synthesis, non-linear optical and electrochemical properties of novel organoimido polyoxometalate derivatives. Ph.D. Dissertation, University of East Anglia, 2016.
- Kennedy, S.R.; Kozar, M.N.; Yennawar, H.P.; Lear, B.J. Synthesis and characterization of the gold dithiolene monoanion, (Bu4N)[Au(pdt=2,3-pyrazinedithiol)2]. Polyhedron 2016, 103, 100–104.
- Pourrieux, G.; Abate, P.O.; Vergara, M.M.; Katz, N.E. Redox-induced linkage isomerization detected in [Ru(NH3)5(NVF)](PF6)2(NVF=N-vinylformamide). Inorg. Chem. Commun. 2016, 66, 90-93.
- Yang, Z.(.; Pazdzior, R.; Yee, J.; Rafferty, S. Reduction potential and heme-pocket polarity in low potential cytochrome b5 of Giardia intestinalis. J. Inorg. Biochem. 2016, 158, 110–114.
- DiMarco, B.N.; O'Donnell, R.M.; Meyer, G.J. Cation-Dependent Charge Recombination to Organic Mediators in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2015, 119, 21599–21604.
- Zhao, Y.; Vargas-Barbosa, N.M.; Strayer, M.E.; McCool, N.S.; Pandelia, M.; Saunders, T.P.; Swierk, J.R.; Callejas, J.F.; Jensen, L.; Mallouk, T.E. Understanding the Effect of Monomeric Iridium(III/IV) Aquo Complexes on the Photoelectrochemistry of IrOx·nH2O-Catalyzed Water-Splitting Systems. J. Am. Chem. Soc. 2015, 137, 8749–8757.
- Pazdzior, R.; Yang, Z.(.; Mesbahuddin, M.S.; Yee, J.; van der Est, A.; Rafferty, S. Low reduction potential cytochrome b5 isotypes of Giardia intestinalis. Exp. Parasitol. 2015, 157, 197-201.
- Bischof, A.M.; Zhang, S.; Meyer, T.Y.; Lear, B.J. Quantitative Assessment of the Connection between Steric Hindrance and Electronic Coupling in 2,5-Bis(alkoxy)benzene-Based Mixed-Valence Dimers. J. Phys. Chem. C 2014, 118, 12693–12699.
- Thomsen, J.M.; Sheehan, S.W.; Hashmi, S.M.; Campos, J.; Hintermair, U.; Crabtree, R.H.; Brudvig, G.W. Electrochemical Activation of Cp* Iridium Complexes for Electrode-Driven Water-Oxidation Catalysis. J. Am. Chem. Soc. 2014, 136, 13826–13834.
- Zhong, F.; Lisi, G.P.; Collins, D.P.; Dawson, J.H.; Pletneva, E.V. Redox-dependent stability, protonation, and reactivity of cysteine-bound heme proteins. Proceedings of the National Academy of Sciences 2014, 111, E306—-E315.
- Navarathne, D.; Skene, W.G. Towards Electrochromic Devices Having Visible Color Switching Using Electronic Push–Push and Push–Pull Cinnamaldehyde Derivatives. ACS Appl. Mater. Interfaces 2013, 5, 12646–12653.
- King, J.D.; McIntosh, C.L.; Halsey, C.M.; Lada, B.M.; Niedzwiedzki, D.M.; Cooley, J.W.; Blankenship, R.E. Metalloproteins Diversified: The Auracyanins Are a Family of Cupredoxins That Stretch the Spectral and Redox Limits of Blue Copper Proteins. Biochemistry (Mosc.) 2013, 52, 8267–8275.
- Kim, J.; Yennawar, H.P.; Lear, B.J. Synthesis and characterization of ruthenium polypyridyl complexes with hydroxypyridine derivatives: effect of protonation and ethylation at the pyridyl nitrogen. Dalton Trans. 2013, 42, 15656-15662.
- Palmer, J.H.; Lancaster, K.M. Molecular Redox: Revisiting the Electronic Structures of the Group 9 Metallocorroles. Inorg. Chem. 2012, 51, 12473–12482.