1. Technique Overview
Linear Sweep Voltammetry (LSV) is a basic potentiostatic sweep method. It is equivalent to a one-segment cyclic voltammetry experiment In LSV, working electrode potential is swept linearly between final and initial values and current is measured as a function of time. The most common output from an LSV experiment is current vs. potential, called a voltammogram.
At its most basic level, LSV sweeps potential vs. reference electrode in one direction, often through the electroactive species’ E0, which allows for the investigation of the resulting electrochemical species generated at the electrode surface. LSV provides both qualitative and quantitative information about electrochemical systems and has become well-established as a fast and reliable characterization tool. LSV is often used to study the kinetics of electron transfer reactions, including catalysis, and has been expanded for use in organic and inorganic synthesis, sensor and biological system evaluation, and fundamental physical mechanics of electron transfer reactions, such as reversibility, formal potentials, and diffusion coefficient determination.
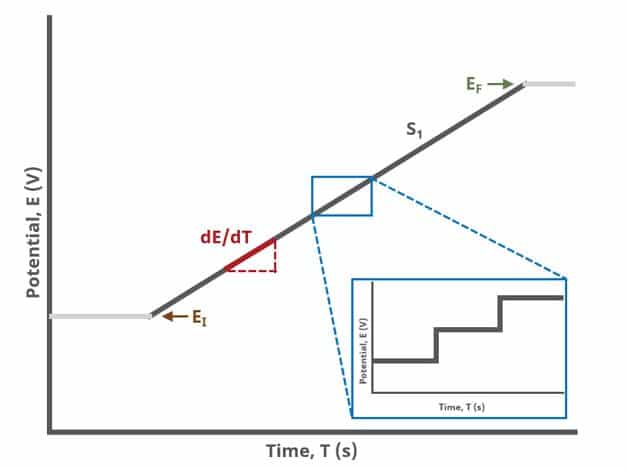
In an LSV experiment, potential is swept linearly from an initial to final potential, sampling current at specified intervals (see Figure 1).
Modern Pine Research potentiostats have digital waveform generators on board. This means that linear sweeps are approximated by a series of small stair steps, whose step size is defined by the 16-bit resolution Analog-to-Digital converter (ADC) on the circuit board and current/potential range selected. For example, on the WaveDriver 100, the current step resolution on the ±100 nA range is
Appropriate current and/or potential filters are automatically employed to “smooth” the jagged edges of this step sequence, enhancing the linearity of the sweep, but can be controlled by the user on the Filters tab of any experiment.
2. Fundamental Equations
A brief summary of the theory of cyclic voltammetry will be covered here. Randles1 and Ševčík2 contributed to the development of the theory for cyclic voltammetry; however, credit for the modern treatment and notation is attributed to Nicholson and Shain3. Additionally, Bard and Faulkner4 provide a nice summary and description of linear sweep voltammetry as do Kissinger and Heinemann5.
Consider a reaction
with a formal potential . If a potential sweep is started sufficiently more positive than
and swept negatively, a non-faradaic current will initially flow. As the potential of the electrode approaches
,
starts reducing to
, which creates a concentration gradient leading to an increased flux (mass transfer) to the surface of the electrode. As
passes
, the concentration of
at the surface of the electrode is nearly zero and mass transfer reaches its maximum. The current begins to tail as the potential is swept to the final potential. The resultant peak height
in amperes (A) is be described by the Randles-Ševčík equation,
where n is the number of electrons, F is Faraday’s Constant (96,485 C/mol), A is the electrode area, D is the diffusion coefficient, C is the concentration, R is the universal gas constant (8.314 J/mol⋅K), T is the absolute temperature (K) and is the sweep rate. At 25°C, the equation simplifies to
As a general approximation,
3. Experimental Setup in AfterMath
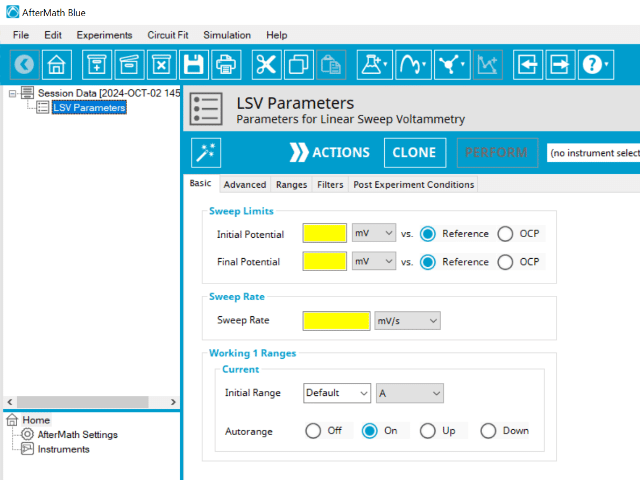
Doing so creates an entry within the archive, called LSV Parameters. In the right pane of the AfterMath application, several tabs will be shown (see Figure 3).
As with many Aftermath methods, the experiment sequence is
Induction Period → Sweep → Relaxation Period → Post-Experiment Idle Conditions
Like many methods, the Induction and Relaxation Periods are on the Advanced Tab. The parameters for a LSV experiment are fairly simple compared to other methods in AfterMath.
In general, enter minimum required parameters on the Basic tab and press “Perform” to run an experiment. AfterMath will perform a quick audit of the parameters you entered to ensure their validity and appropriateness for the chosen instrument, followed by the initiation of the experiment. In some cases, users may desire to adjust additional settings such as filters, post- experiment conditions, and post-experiment processing before clicking the “Perform” button. Continue reading for detailed information about the fields on each unique tab.
3.1. Basic Tab
Click the AutoFill button on the top bar in AfterMath to automatically fill all required parameters with reasonable starting values. While the values provided may not be appropriate for your specific system, they are reasonable parameters with which to start your experiment, especially if you are new to the method.
The basic tab contains fields for the fundamental parameters necessary to perform an LSV experiment. AfterMath shades fields with yellow when a required entry is blank and shades fields pink when the entry is invalid (see Figure 4).
During the induction period, a set of initial conditions are applied to the electrochemical cell and the cell equilibrates at these conditions (set on the Advanced Tab). Data are not collected during the induction period, nor are they shown on the plot during this period.
After the induction period, the potential applied to the working electrode is swept to the next specified value (based on segments) for the duration of the experiment, which is called the sweep period. The potentiostatic circuit of the instrument maintains control over increasing potential while simultaneously measuring the current at the working electrode relative to the reference electrode. During the sweep segments, potential and current at the working electrode are recorded at regular intervals as specified on the Advanced Tab.
The experiment concludes with a relaxation period. During the relaxation period, a set of final conditions (specified on the Advanced tab) are applied to the electrochemical cell and the cell equilibrates at these conditions (set on the Advanced Tab). Data are not collected during the induction period, nor are they shown on the plot during this period.
A plot of the typical experiment sequence, containing labels of the fields on the Basic tab, helps to illustrate the sequence of events in an LSV experiment (see Table 1 and Figure 5).
The table below lists the group and field names and symbol for each parameter associated with this experiment (see Table 1).
The Electrode Range group on the Basic Tab is used to specify the expected potential and/or current range to use on the experiment. For LSV, current is the measured valued and as such, users can select the most appropriate current range from the dropdown menu. The most appropriate range is the one that completely includes the expected current spread across the entire experiment, but it not significantly greater. Note that the selection chooses the initial range. If Autorange is not Off, then as data are collected, AfterMath will choose the most appropriate range as needed. If Autorange is Off, and the initial range is too small, then current may go off scale and the results will be truncated. If the initial current range is too large, and Autorange is Off, then the data may have a noisy, choppy, or quantized appearance.
At the end of the relaxation period, the post-experiment idle conditions are applied to the cell, and the instrument returns to the idle state. The default plot generated from the data is measured current vs. potential, called a voltammogram.
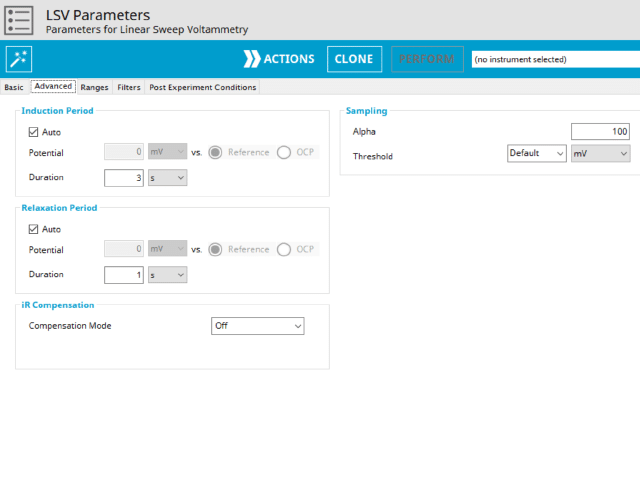
3.2. Advanced Tab
The LSV Advanced tab contains groups for Induction Period, Relaxation Period, iR Compensation, and Sampling (see Figure 5).
Induction Period is the first step in an LSV experiment if the Duration is >0 s. During the induction period, the specified current is applied to the cell for the specified duration. During this period, data are not collected. The Induction Period is believed to “calm” the cell prior to intentional perturbation.
Relaxation Period is the last step in a LSV experiment if the Duration is >0 s. During the relaxation period, the specified current is applied to the cell for the specified duration. During this period, data are not collected. The Relaxation Period is believed to “calm” the cell after intentional perturbation.
iR compensationis used to correct for uncompensated resistance in the electrochemical cell.
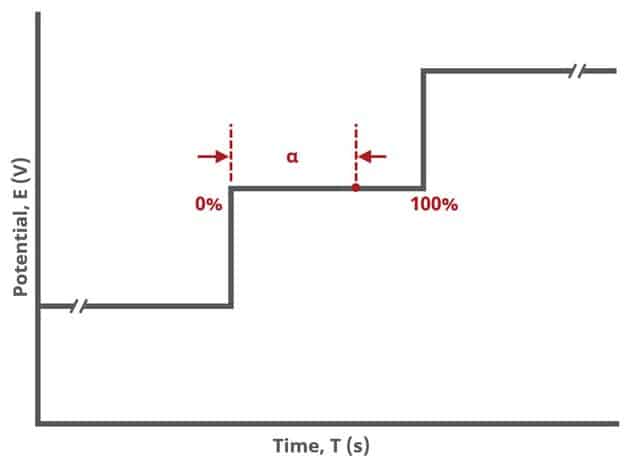
The Sampling group defines the potential sampling rate for the experiment There are two parameters in this group, Alpha and Threshold. As mentioned previously, digital instruments (such as the WaveNow and WaveDriver series potentiostats) approximate a linear sweep with a series of tiny steps.
As shown, alpha is the total time after each small step, from 0% to 100% and the value of alpha defines the time at which a sample is measured (see Figure 6). If , current is measured immediately after the small step. If
then current is measured just before the next small step. In general, it is recommended to measure sweep experiments at \alpha=100, which is the default setting.
Threshold defines the frequency of sampling. There are two options from the dropdown which are “Default” and “None.” Additionally, a specific current interval can be added by typing a numeric value into the dropdown box and selecting the appropriate units. Briefly, the options can be described as follows:
- Default. This setting will use the default settings, which are 5 mV for potentiostatic experiments and 1 µA for galvanostatic experiments.
- None. This setting will enable the collection of the maximum number of data points possible. The value is not easily known, as is a combination of the sweep rate and sweep limits. Choose this option to collect the maximum number of data points that the hardware can acquire.
- Manual entry. In this case, type an integer value into the dropdown menu and select the appropriate units in the next dropdown menu. For example, a user may only wish to collect a data point every 20 mV, in which case these are entered into the fields.
Not all threshold values, manually entered, will be allowed. The software will use the value provided and match as closely to it as possible. The actual sampling rate may be higher, exact, or lower than the value entered. Presently, AfterMath will not inform you of the threshold value actually possible, but it can be inferred after the completion of the experiment and viewing the time-based data stream, where the threshold can be calculated as the time difference between data points.
Archive file size increases as the threshold decreases. In other words, a smaller threshold means more data will be collected per experiment whereas a larger threshold means fewer data will be collected per experiment. For long term experiments, Pine Research recommends the default threshold, unless users require a finer level of detail per sweep.
Lastly, the iR Compensation group allows users to adjust the cell feedback to accommodate a known resistive drop between working and reference electrodes. Not all potentiostats from Pine Research support iR compensation. The WaveDriver series and WaveNow Wireless support iR compensation by positive feedback. The WaveNow, WaveNano, WaveNowXV and the CBP bipotentiostat do not support iR compensation of any type. More information about iR compensation, including understanding how it works and how to determine the resistance, consult the support article on the topic.
3.3. Ranges, Filters, and Post Experiment Conditions Tab
In nearly all cases, the groups of fields on the Ranges tab are already present on the Basic tab. The Ranges tab shows an Electrode Range group and depending on the experiment shows either, or both, current and potential ranges and the ability to select an autorange function. The fields on this tab are linked to the same fields on the Basic tab (for most experiments). Changing the values on either the Ranges tab or on the Basic tab changes the other set. In other words, the values selected for these fields will always be the same on the Ranges tab and on the Basic tab. More on ranges is found within the website.
The Filters tab provides access to potentiostat hardware filters, including stability, excitation, current response, and potential response filters. Pine Research recommends that users contact us for help in making changes to hardware filters. Advanced users may have an easier time changing the automatic settings on this tab.
By default, the potentiostat disconnects from the electrochemical cell at the end of an experiment. There are other options available for what these post-experiment conditions can be and are controlled by setting options on the Post Experiment Conditions tab.
4. Example Applications
Most applications of linear sweep voltammetry relate to cyclic voltammetry. However, here are a few examples where linear sweep voltammetry was applied.
In the first example, Cheng and coworkers used linear sweep voltammetry to examine direct methane production using a biocathode containing methanogens in either an electrochemical system or a microbial electrolysis cell by a process called electromethanogenesis6. Since the production of methane from CO2 is an irreversible process, cyclic voltammetry would provide no additional benefit over linear sweep voltammetry. Linear sweep voltammetry was used to show that a biocathode produced higher current densities than a plain carbon cathode. The authors were able to show that methane can be produced directly from an electrical current without hydrogen gas.
In another example, Wang and coworkers used linear sweep voltammetry to examine the release of inorganic ions and DNA from an ionorganic ion/DNA bilayer film7. Performing linear sweep voltammetry simultaneously with surface plasmon resonance, the researchers were able to show that the film disassembled upon sweeping the potential to more negative values. This allowed the researchers to demonstrate the controlled release of DNA for gene-targeting therapy.
5. References
- Randles, J. E. B. A cathode ray polargraph. Part II – The current-voltage curves. Trans. Faraday Soc., 1948, 44, 327-338.
- Ševčík, A. Oscillographic Polarography with Periodical Triangular Voltage. Collect. Czech. Chem. Commun., 1948, 1948, 349-377.
- Nicholson, R. S.; Shain, I. Theory of Stationary Electrode Polarography. Single Scan and Cyclic Methods Applied to Reversible, Irreversible, and Kinetic Systems. Anal. Chem., 1964, 36(4), 706-723.
- Bard, A. J.; Faulkner, L. A. Electrochemical Methods: Fundamentals and Applications, 2nd ed. Wiley-Interscience: New York, 2000.
- Kissinger, P.; Heineman, W. R. Laboratory Techniques in Electroanalytical Chemistry, 2nd ed. Marcel Dekker, Inc: New York, 1996.
- Cheng, S.; Xing, D.; Call, D. F.; Logan, B. E. Direct Biological Conversion of Electrical Current into Methane by Electromethanogenesis. Environ. Sci. Technol., 2009, 43(10), 3953-3958.
- Wang, F.; Li, D.; Li, G.; Liu, X.; Dong, S. Electrodissolution of Inorganic Ions/DNA Multilayer Film for Tunable DNA Release. Biomacromolecules, 2008, 9(10), 2645-2652.