Empirical Determination of RRDE Collection Efficiency (N)
Last Updated: 1/5/23 by Alex Peroff
1General Overview
Direct computation of the theoretical collection efficiency is possible using the theoretical relationships
 Theoretical Determination of Collection Efficiency (N)
if the actual machined dimensions of the disk and ring are known for a particular RRDE. In practice, the actual RRDE dimensions may not be known due to uncertainties in the machining process and changes in the dimensions induced by electrode polishing or temperature cycling. For this reason, it is common practice to empirically measure the collection efficiency using a well-behaved redox system rather than to rely upon a computed value.
Theoretical Determination of Collection Efficiency (N)
if the actual machined dimensions of the disk and ring are known for a particular RRDE. In practice, the actual RRDE dimensions may not be known due to uncertainties in the machining process and changes in the dimensions induced by electrode polishing or temperature cycling. For this reason, it is common practice to empirically measure the collection efficiency using a well-behaved redox system rather than to rely upon a computed value.
The ferrocyanide/ferricyanide half reaction is a simple, single-electron, reversible half reaction that is often used as the basis for measuring collection efficiency.
 Paulus, U. A.; Schmidt, T. J.; Gasteiger, H. A.; Behm, R. J. Oxygen reduction on a high-surface area Pt/Vulcan carbon catalyst: A thin-film rotating ring-disk electrode study. J. Electroanal. Chem., 2001, 495(2), 134–145.
The RRDE is placed in a solution containing a small concentration (~10 mM) of potassium ferricyanide, K3Fe(CN)6 , in a suitable aqueous electrolyte solution (such as 1.0 M potassium nitrate, KNO3) and is operated at rotation rates between 500 and 2000 RPM. Initially, both the ring and the disk electrodes are held at a sufficiently positive potential that no reaction occurs. Then, the potential of the disk electrode is slowly swept (~50 mV/sec) towards more negative potentials, and a cathodic current is observed which corresponds to the reduction of ferricyanide to ferrocyanide at the disk.
Paulus, U. A.; Schmidt, T. J.; Gasteiger, H. A.; Behm, R. J. Oxygen reduction on a high-surface area Pt/Vulcan carbon catalyst: A thin-film rotating ring-disk electrode study. J. Electroanal. Chem., 2001, 495(2), 134–145.
The RRDE is placed in a solution containing a small concentration (~10 mM) of potassium ferricyanide, K3Fe(CN)6 , in a suitable aqueous electrolyte solution (such as 1.0 M potassium nitrate, KNO3) and is operated at rotation rates between 500 and 2000 RPM. Initially, both the ring and the disk electrodes are held at a sufficiently positive potential that no reaction occurs. Then, the potential of the disk electrode is slowly swept (~50 mV/sec) towards more negative potentials, and a cathodic current is observed which corresponds to the reduction of ferricyanide to ferrocyanide at the disk.
As ferricyanide is reduced at the disk electrode, the ferrocyanide generated by this process is swept outward (radially) away from the disk electrode and toward the ring electrode. The ring electrode is held constant at a positive (oxidizing) potential throughout the experiment. Some (but not all) of the ferrocyanide generated at the disk travels close enough to the ring electrode that it is oxidized back to ferricyanide. Thus, an anodic current is observed at the ring electrode due to the oxidation of ferrocyanide to ferricyanide at the ring.
The measured ratio of the ring (anodic) limiting current to the disk (cathodic) limiting current is the empirical collection efficiency. As the rotation rate increases, both the disk and the ring currents increase (see Figure 1). Because both the anodic and cathodic limiting currents are proportional to the square root of the rotation rate, the empirical collection efficiency is expected to be independent of the rotation rate.
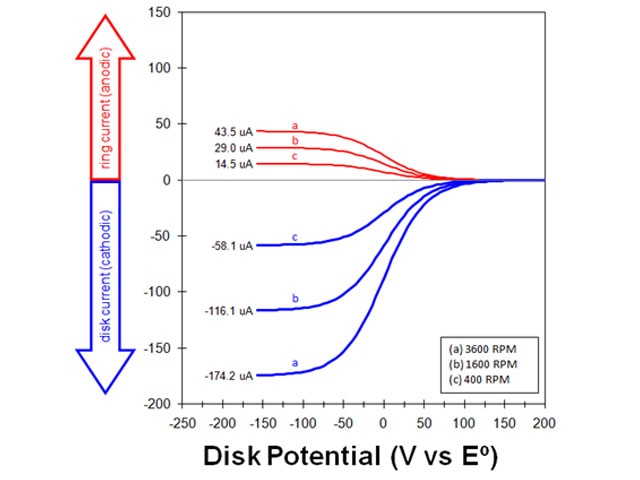
Figure 1. rotating ring-disk electrode voltammograms at various rotation rates
Once the collection efficiency value has been established empirically for a particular RRDE, it can be treated as a property of that particular RRDE, even if the RRDE is used to study a different half reaction in a different solution on a different day. Although the empirically measured collection efficiency (Nempirical) is a ratio of two currents which often have opposite mathematical signs (anodic and cathodic), the empirical collection efficiency is always expressed as a positive number, as follows,
where nD and nR are the number of electrons exchanged at the disk and ring (and very often, nD and nR are equal to each other).



