This WaveDriver® 200 Electrochemical Workstation is a versatile, dual-electrode, research-grade, performance-driven system with potentiostat, galvanostat, EIS, open-circuit potential, and zero resistance ammeter modes of operation. It is engineered to provide the essential hardware and software features you need at an affordable price. This system finds use with stationary electrodes, rotating disk (RDE), rotating ring-disk (RRDE), and rotating cylinder (RCE) electrodes.
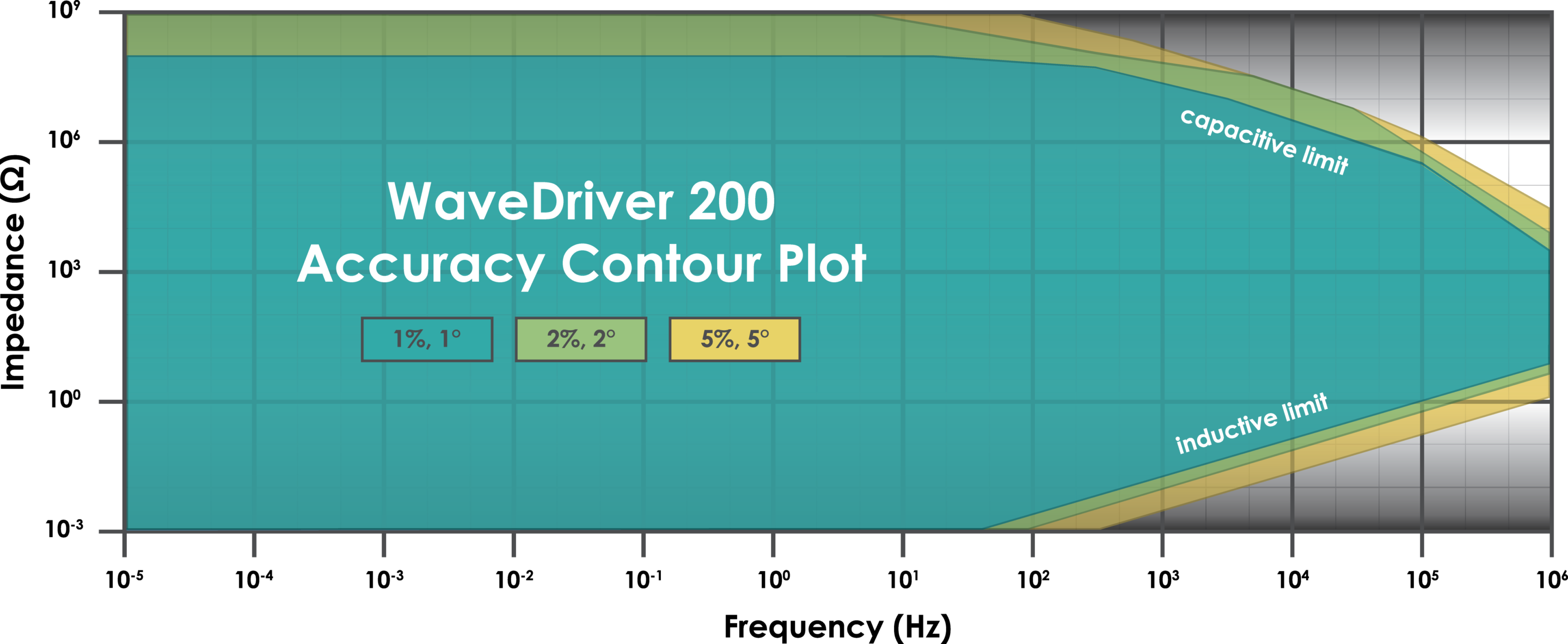
Under the control of our powerful AfterMath® Blue software package, the WaveDriver 200 supports a wide range of DC electroanalytical techniques as well as AC techniques like Electrochemical Impedance Spectroscopy (EIS). It offers current ranges up to ±1 A, potential ranges up to ±15 V, advanced inputs/outputs, waveform filters, EIS up to 1 MHz, and iR compensation.
The WaveDriver 200 is a true integrated bipotentiostat, capable of controlling one or two working electrodes operating in the same electrochemical cell along with a counter and reference electrode, making this instrument ideal for Rotating Ring-Disk Electrode (RRDE) voltammetry. This workstation is a trusted companion to our popular electrode rotators, including our latest evoLUTION, the MSR evo Electrode Rotator.
WaveDriver 200 Electrochemical Workstation
The WaveDriver 200 Electrochemical Workstation is an excellent choice. Unlike other "bipotentiostats" on the market, this workstation electronics features a truly integrated bipotentiostat. You do not have to mess around with cell cables in obscure configurations to operate the workstation as a bipotentiostat. It is ready to use out of the box - just connect your clearly-marked cell cable leads to the desired electrodes and you are ready to go with your experiment, like rotating ring-disk (RRDE). No additional cables, adapters, or connectors required.
The WaveDriver 200 Electrochemical Workstation is engineered to provide the essential hardware and software features you need at an affordable price. It supports a wide range of DC electroanalytical techniques as well as AC techniques such as Electrochemical Impedance Spectroscopy (EIS). It offers current ranges up to ±1 A, potential ranges up to ±15 V, advanced inputs/outputs, waveform filters, EIS up to 1 MHz, and iR compensation. The WaveDriver 200 is a high performance, EIS-capable workstation with integrated bipotentiostat, which is ideal for use with stationary electrodes, RDE, RRDE, and RCE.
AfterMath Blue - Data Acquisition, Analysis, and Organization Software
Licenses for our AfterMath® Blue software are included with the purchase of an Electrochemical Workstation. The licenses give you access to all of the AC and DC methods that the WaveDriver 200 can perform. Pine Research does not charge extra for various categories of electrochemical techniques. If the instrument is capable of a method, you have access to it (no hidden costs). Here are some unique features of AfterMath Blue:
- Instrument Control. When started, AfterMath automatically detects all compatible instrumentation attached to the computer and provides complete control over each instrument. It can simultaneously control multiple instruments and queue multiple experiments.
- Flexible Plotting. AfterMath offers a powerful "drag-n-drop" feature for quickly copying and moving traces between plots, with precise control over various plot settings.
- Scientific Units. Designed with scientific data in mind, AfterMath properly handles units, metric prefixes, and significant figures.
- Data Archiving. A unique XML-based file format allows multiple related experiments to be stored in a single archive file, simplifying file management.
- Tools and Transforms. Flexible tools allow precise measurement of quantities like peak height and area, with fundamental mathematical operations available on any trace.
Our talented team of engineers and chemists have taken a careful approach at integrating EIS into our Electrochemical Workstations. Our software engineering team has incorporated EIS equivalent circuit fitting directly into our AfterMath Blue software (no separate software required). Multiple curve fitting algorithms and options allow you to fit even the most troublesome data. Here are some of the exciting and novel EIS analysis features in AfterMath Blue:
- Integrated Curve Fitting and Analysis. Our software team has seamlessly integrated EIS curve fitting into AfterMath. Why work with more than one software application to fit your EIS data when you can do it using the very same software application that acquired the data? AfterMath EIS curve fitting utilities provide several analyses, including Circuit Fit, Transmission Line, and Kramers-Kronig. Unlike others, our fitting software also provides several fitting methods including Modified Levenberg-Marquardt (LM), Simplex, and Powell algorithms in addition to fitting options that include dynamic point selection, unity, and parametric fitting.
- Novel Transmission Line Fitting. AfterMath provides a unique approach to model your porous electrodes. While the transmission line model is not new, AfterMath provides you with some unique transmission line fitting tools. Instead of a static circuit, where you have no control over the elements of the model, we provide a very flexible basic model, from which you can customize nearly all aspects of the model to suit your system. Give it a try - import your three- or five-column EIS data directly into AfterMath and see the difference with our transmission line fitting.
- Finishing Touches. While fitting your EIS data, why flip back and forth between Nyquist and Bode plots? Why not be able to view both plots and fits simultaneously? We heard this feedback from many customers and have designed AfterMath to provide you with both plots simultaneously during fitting. You can also draw your own equivalent circuits to fit your particular system.
- Pandey, S.; Dubey, S.; Singh, V.; Ganesan, V. Vanadium-Doped FeNiSe4 as a High-Performance Oxygen Evolution Electrocatalyst: Synergistic Effects of Se–Ni–Fe Hybridization and Vanadium-Induced Electronic Modulation. ACS Applied Energy Materials 2025.
- Wagner, A.A.; Clarke, T.B.; Perez Herrera, D.; Thompson, D.H.; Payne, E.M.; Dick, J.E. Considerations for Accurate Soft Particle Sizing Using Stochastic Electrochemistry. ACS Electrochemistry 2025.
- Meunier, C.J.; McCarty, G.S.; Sombers, L.A. Tracking Carbon Microelectrode Impedance during Fast-Scan Cyclic Voltammetry. ACS Sens. 2025, 10, 3617-3627.
- Todorov, J.; McCarty, G.S.; Sombers, L.A. Mechanistic Insight into Tyrosine Oxidation at Carbon-Fiber Microelectrodes Revealed by Fast-Scan Cyclic Voltammetry. ACS Electrochemistry 2025.
- Li, H.; Hansen, L.; Aliyeva, A.; Wang, J.; Qiu, H.; Müller, M.; Chen, S.; Aktas, C.; Kienle, L.; Hartke, B.; Benedikt, J. Plasma-engineering of Pt-decorated NiCo2O4 nanowires with rich oxygen vacancies for enhanced oxygen electrocatalysis and zinc-air battery performance. Applied Catalysis B: Environment and Energy 2025, 361, 124607.
- Bredar, A.R.C.; Margavio, H.R.M.; Donley, C.; Spinner, N.; Amin, N.; Parsons, G.N.; Dempsey, J.L. Oxidation Temperature-Dependent Electrochemical Doping of WO3 Deposited via Atomic Layer Deposition. The Journal of Physical Chemistry C 2024, 128, 21539-21550.
- Jeon, H.; Jo, H.; Seo, S.; Lee, S.; Yoon, S.; Han, D. In-situ spectroelectrochemical analysis: Irreversible deformation of cesium lead bromide Perovskite Quantum Dots in SiOx matrices. Sensors and Actuators Reports 2024, 8, 100208.
- Lyu, X.; Yang, J.; Serov, A. Is Pt dissolution a concern from the counter electrode in electrochemical oxygen evolution reaction?. Electrochim. Acta 2024, 501, 144824.
- Isaacs, D.P.; Dempsey, J.L. Synthesis and characterization of a series of CpW(CO)2PR3H, [CpW(CO)2PR3]−, [CpW(CO)2PR3(CH3CN)]+, and [CpW(CO)2PR3]2 complexes. Inorg. Chim. Acta 2024, 571, 122238.
- Zuccante, G.; Acciarri, M.; Vecchio, C.L.; Gatto, I.; Baglio, V.; Pianta, N.; Ruffo, R.; Navarini, L.; Santoro, C. Oxygen reduction reaction platinum group metal-free electrocatalysts derived from spent coffee grounds. Electrochim. Acta 2024, 492, 144353.
- Ngozichukwu, B.; Pranada, E.; Johnson, D.; Djire, A. Nanolayered Ti4N3Tx MXene Retains Its Electrocatalytic Properties after Prolonged Immersion in Solvents. ACS Appl. Nano Mater. 2024, 7, 13765-13774.
- Ahmed, S.I.U.; Sankarasubramanian, S. Low pH Titanium Electrochemistry in the Presence of Sulfuric Acid and its Implications for Redox Flow Battery Applications. J. Electrochem. Soc. 2024, 171, 060538.
- Xue, F.; Fu, X.; Kang, S.; Sheng, X.; Li, B.; Shen, P.K.; Zhu, J.; Nie, M.; Lu, S.; Lu, W. Mo-Based MXene-Supported Pt Nanoparticles for Highly Durable Oxygen Reduction in Acidic Electrolytes. ACS Appl. Nano Mater. 2024, 7, 6305-6314.
- Zhang, P.; Chen, H.; Zhu, H.; Chen, K.; Li, T.; Zhao, Y.; Li, J.; Hu, R.; Huang, S.; Zhu, W.; Liu, Y.; Pan, Y. Inter-site structural heterogeneity induction of single atom Fe catalysts for robust oxygen reduction. Nat. Commun. 2024, 15, 2062.
- Forderhase, A.G.; Ligons, L.A.; Norwood, E.; McCarty, G.S.; Sombers, L.A. Optimized Fabrication of Carbon-Fiber Microbiosensors for Codetection of Glucose and Dopamine in Brain Tissue. ACS Sens. 2024, 9, 2662-2672.
- Zuccante, G.; Muhyuddin, M.; Ficca, V.C.A.; Placidi, E.; Acciarri, M.; Lamanna, N.; Franzetti, A.; Zoia, L.; Bellini, M.; Berretti, E.; Lavacchi, A.; Santoro, C. Transforming Cigarette Wastes into Oxygen Reduction Reaction Electrocatalyst: Does Each Component Behave Differently? An Experimental Evaluation. ChemElectroChem 2024, 11, e202300725.
- Islam, T. Iron Phthalocyanine Functionalized Boron Doped Graphene as an Inexpensive Cathode Catalyst for Alkaline Fuel Cells - ProQuest. Master's Thesis, New Mexico Institute of Mining and Technology, 2024.
- Lyu, X.; Bai, Y.; Li, J.; Tao, R.; Yang, J.; Serov, A. Investigation of oxygen evolution reaction with 316 and 304 stainless-steel mesh electrodes in natural seawater electrolysis. J. Environ. Chem. Eng. 2023, 11, 109667.
- Lin, C.; Zhang, H.; Zhang, X.; Liu, Y.; Zhang, Y. Kinetics-Driven MnO2 Nanoflowers Supported by Interconnected Porous Hollow Carbon Spheres for Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 14388-14398.
- Cetindere, S.; Ardic Alidagi, H.; Anjass, M. Two novel Anderson-type polyoxometalate based MnIII complexes constructed from pyrene derivatives: Synthesis, photophysical, and electrochemical properties. Inorg. Chim. Acta 2023, 545, 121280.
- Lu, X.; You, W.; Peltier, C.R.; Coates, G.W.; Abruña, H.D. Influence of Ion-Exchange Capacity on the Solubility, Mechanical Properties, and Mass Transport of Anion-Exchange Ionomers for Alkaline Fuel Cells. ACS Applied Energy Materials 2023, 6, 876-884.
- Lin, C.; Liu, Y.; Zhang, X.; Miao, X.; Chen, Y.; Chen, S.; Zhang, Y. Regulating the plating process of zinc with highly efficient additive for long-life zinc anode. J. Power Sources 2022, 549, 232078.
- Raj, S.K.; , .; Sharma, V.; Srivastava, D.N.; Kulshrestha, V. Single-Step Synthesis of Well-Ordered Hierarchical Nickel Nanostructures for Boosting the Oxygen Evolution Reaction. Energy Fuels 2022, 36, 13786-13795.
- Molodtsova, T.; Gorshenkov, M.; Kubrin, S.; Saraev, A.; Ulyankina, A.; Smirnova, N. One-step access to bifunctional γ-Fe2O3/δ-FeOOH electrocatalyst for oxygen reduction reaction and acetaminophen sensing. J. Taiwan Inst. Chem. Eng. 2022, 140, 104569.
- Guo, Q.; Li, H.; Wang, S.; Gong, Y.; Ren, L.; Yu, G. Experimental study on preparation of oxygen reduction catalyst from coal gasification residual carbon. Chemical Engineering Journal 2022, 446, 137256.
- Xu, W.; Yoon, D.; Yang, Y.; Xiong, Y.; Li, H.; Zeng, R.; Muller, D.A.; Abruña, H.D. MOF-Derived Bimetallic Pd–Co Alkaline ORR Electrocatalysts. ACS Appl. Mater. Interfaces 2022, 14, 44735-44744.
- Lyu, X.; Li, J.; Jafta, C.J.; Bai, Y.; Canales, C.P.; Magnus, F.; Ingason, Á.S.; Serov, A. Investigation of oxygen evolution reaction with Ni foam and stainless-steel mesh electrodes in alkaline seawater electrolysis. J. Environ. Chem. Eng. 2022, 10, 108486.
- Wu, S.; Yang, J.; Qin, N.; Li, Y.; Wang, H.; Zhang, Y.; Wang, Q.; Lu, Z. Ethyl Viologen as a Superoxide Quencher to Enhance the Oxygen Reduction Reaction in Li–O2 Batteries. ACS Applied Energy Materials 2022, 5, 9040-9048.
- Li, X.; Mu, W.; Chen, B.; He, Y.; Tu, J.; Yang, Y.; Yang, Y.; Wei, H.; Peng, S. Complexation of uranyl with chelidamic acid: Crystal structures, binding strength, and electrochemical redoxes. Nuclear Analysis 2022, 1, 100014.
- Wang, Y.; Chen, Y.; Wang, Z.; Li, P.; Zhao, J.; Zhao, H.; Li, D.; He, T.; Wei, Y.; Su, Y.; Xiao, C. Boron doping induced electronic reconfiguration of Fe-Nx sites in N-doped carbon matrix for efficient oxygen reduction reaction in both alkaline and acidic media. Int. J. Hydrogen Energy 2022, 47, 18663-18674.
- Lee, S.J.; Lee, Y.J.; Seo, S.; Jeon, H.; Han, D.; Im, H.; Shrestha, N.K.; Yoon, S.J. Insulating CsPbBr3 Quantum Dots via Encapsulation with SiOx: Interfacial Electron Trafficking and Interaction beyond the Insulating Boundary. The Journal of Physical Chemistry C 2022, 126, 7910-7921.
- Testa, D.; Zuccante, G.; Muhyuddin, M.; Landone, R.; Scommegna, A.; Lorenzi, R.; Acciarri, M.; Petri, E.; Soavi, F.; Poggini, L.; Capozzoli, L.; Lavacchi, A.; Lamanna, N.; Franzetti, A.; Zoia, L.; Santoro, C. Giving New Life to Waste Cigarette Butts: Transformation into Platinum Group Metal-Free Electrocatalysts for Oxygen Reduction Reaction in Acid, Neutral and Alkaline Environment. Catalysts 2022, 13, 635.
- Lee, S.J.; Lee, Y.J.; Seo, S.; Jo, H.; Han, D.; Yoon, S.J. Effect of the surroundings on the photophysical properties of CsPbBr3 perovskite quantum dots embedded in SiOx matrices. Bull. Korean Chem. Soc. 2022, 43, 1312-1319.
- Clark, R.B.; Glasscott, M.W.; Verber, M.D.; DeMartino, J.C.; Netchaev, A.; Ray, J.D.; Brown, E.W.; Alberts, E.; Fernando, P.U.A.I.; Moores, L.C.; Dick, J.E. A Generalized Potentiostat Adaptor for Multiplexed Electroanalysis. Anal. Chem. 2021, 93, 7381-7387.
- Miao, X.; Zhang, X.; Chen, S.; Liu, Y.; Chen, Y.; Lin, J.; Chen, Q.; Zhang, Y. Dual-redox enhanced supercapacitors with sodium anthraquinone-2-sulfonate and potassium bromide. Electrochim. Acta 2021, 374, 137889.
- Askari, S.; Mariotti, D.; McGlynn, R.; Benedikt, J. Air-Cathode with 3D Multiphase Electrocatalyst Interface Design for High-Efficiency and Durable Rechargeable Zinc–Air Batteries. Energy Technology 2021, 9, 2000999.
- Osipova, D. Nanostructured carbon from biomass as a catalyst for energy conversion devices. Master's Thesis, Aalto University, 2021.
- Lee, C.; Kim, K.; Shin, Y.; Han, D.; Yoon, S.J. In Situ Spectroelectrochemical Investigation of Perovskite Quantum Dots for Tracking Their Transformation. Frontiers in Energy Research 2021, 8, -.
- Yi, X.; Yin, F.; He, X.; Li, G. Partially reduced NiO by cellulose as a highly active catalyst for oxygen evolution reaction: synergy between in situ generated Ni3+ and lattice oxygen. International Journal of Energy Research 2021, 45, 15544-15556.
- Narulkar, D.D.; Devulapally, K.; U, A.K.; Dhuri, S.N.; Dhavale, V.M.; Vardhaman, A.K.; Giribabu, L. A novel nonheme manganese(II) complex for (electro) catalytic oxidation of water. Sustainable Energy & Fuels 2020, 4, 2656-2660.
- Meunier, C.J.; Denison, J.D.; McCarty, G.S.; Sombers, L.A. Interpreting Dynamic Interfacial Changes at Carbon Fiber Microelectrodes Using Electrochemical Impedance Spectroscopy. Langmuir 2020, 36, 4214-4223.
- Brown, C.A. Insertion and Frustrated Lewis Pair Chemistry of Rhenium (III) and Rhenium (V) Alkyl and Hydride Complexes. Ph.D. Dissertation, North Carolina State University, 2020.
- Eom, C.J. In Situ Spectroscopy of Metal Oxides Reveal Electrocatalyst Structure-Property Relationships. Ph.D. Dissertation, Cornell University, 2020.
- Yang, Y.; Zeng, R.; Xiong, Y.; DiSalvo, F.J.; Abruña, H.D. Cobalt-Based Nitride-Core Oxide-Shell Oxygen Reduction Electrocatalysts. J. Am. Chem. Soc. 2019, 141, 19241-19245.
- Eom, C.J.; Suntivich, J. In Situ Stimulated Raman Spectroscopy Reveals the Phosphate Network in the Amorphous Cobalt Oxide Catalyst and Its Role in the Catalyst Formation. The Journal of Physical Chemistry C 2019, 123, 29284-29290.
- Zhu, Y. High Temperature CO2RR on Yttrium doped Barium Zirconate Electrolysis Cell. Ph.D. Dissertation, Cornell University, 2019.
- Fehr, J.M.; McKenas, C.G.; Liu, B.; Lockett, M.R. Azide‑alkyne click reactions to prepare chemically modified amorphous carbon electrodes. Appl. Surf. Sci. 2019, 480, 1109-1115.
- Glasscott, M.W.; Pendergast, A.D.; Goines, S.; Bishop, A.R.; Hoang, A.T.; Renault, C.; Dick, J.E. Electrosynthesis of high-entropy metallic glass nanoparticles for designer, multi-functional electrocatalysis. Nat. Commun. 2019, 10, 1-8.
- Liu, H.; Xu, C.; Du, Y.; Ma, F.; Li, Y.; Yu, J.; Zhen, L. Ultrathin Co9S8 nanosheets vertically aligned on N,S/rGO for low voltage electrolytic water in alkaline media. Sci. Rep. 2019, 9, 1951.
- Goines, S.; Dick, J.E. Electrochemical Characterization of Nicotinamide Riboside. ChemElectroChem 2019, 6, 5264-5272.
- Chen, J.; Zhao, G.; Chen, Y.; Rui, K.; Mao, H.; Dou, S.X.; Sun, W. Iron-Doped Nickel Molybdate with Enhanced Oxygen Evolution Kinetics. Chemistry – A European Journal 2019, 25, 280-284.
- Yarur, F.; Manioudakis, J.; Naccache, R.; Majewski, M. Carbon Dot Sensitized Photoanodes for Visible Light Driven Organic Transformations. ChemRxiv 2019, 2, -.
Related Products and Accessories
-

MSR evo Electrode Rotator
AFMSR24B1 -

Reference Electrode, Silver/Silver Chloride, Single Junction
RREF0021 -

WaveVortex 10 Electrode Rotator
AF01WV10 -

E6R1 Series ChangeDisk Rotating Ring-Disk Electrode, Platinum (Pt) Ring, PTFE shroud
AFE6R1PT -

RDE/RRDE Disk Insert, 5 mm OD x 4 mm thick, Glassy Carbon (GC)
AFED050P040GC -
Rotating Disk/Ring-Disk Cell Kit, Standard Volume, Water Jacket
AKCELL3 -

E5/E6 Series ChangeDisk Electrode Toolkit
AFE6K050