Half-Reactions
Last Updated: 4/24/19 by Support
1
Regardless of the electrode geometry being used, the common theme is that an ion or molecule is being conveyed to the electrode surface, and upon arrival, it is either oxidized or reduced depending upon the potential applied to the electrode. If a sufficiently positive potential is applied to the electrode, then the molecules (or ions) tend to be oxidized, and conversely, if a sufficiently negative potential is applied to the electrode, the molecules (or ions) tend to be reduced.
Reduction at a working electrode implies that electrons are being added to the ion or molecule by flowing out of the electrode and into the solution. A current travelling in this direction is said to be a cathodic current. The general form of a reduction half-reaction occurring at an electrode may be written as follows:
| (1) |
where n is the total number of electrons added to the molecule (or ion) when it is converted from the oxidized form (O) to the reduced form (R).
Oxidation at a rotating electrode implies that electrons are being removed from an ion or molecule and are travelling out of the solution and into the electrode. A current travelling in this direction is said to be an anodic current, and the oxidation occurring at the electrode can be represented by the following redox half reaction,
| (2) |
Given that electrochemical half reactions can occur in either direction, they are often written using chemical equilibrium notation as follows:
| (3) |
Each half-reaction has an associated standard electrode potential (E0) which is a thermodynamic quantity related to the free energy associated with the equilibrium. Like many other standard thermodynamic quantities, the standard electrode potential corresponds to a given standard state. The standard state corresponds to a thermodynamic system where the activities of 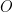 and
and 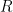 are unity (i.e., when all solution concentrations are 1.0 mol/L, all gases are present at 1.0 atm partial pressure, and other materials are present as pure phases with unity activity).
are unity (i.e., when all solution concentrations are 1.0 mol/L, all gases are present at 1.0 atm partial pressure, and other materials are present as pure phases with unity activity).
To account for the possibility of non-unity activities, the Nernst equation (see below) can be used to express the equilibrium electrode potential (ENERNSTIAN) in terms of the actual activities,
| (4) |
where T is the temperature (K), F is the Faraday constant (F = 96485 C / mol), and R is the ideal gas constant (R = 8.3145 J /mol K). Usually, the activities of molecules or ions dissolved in solution are assumed to be the same as their molar concentrations, so the Nernst Equation is often written as
| (5) |
where CO and CR are the concentrations of the dissolved molecules or ions in the oxidized and reduced forms, respectively, at the surface of the electrode. Note that any liquid or solid phase materials at the electrode surface (such as the solvent or the electrode itself) have unity activity and thus do not appear in the Nernst equation.
This half reaction at an electrode can be driven in the cathodic (reducing) direction by applying a potential to the electrode (EAPPLIED) which is more negative than the equilibrium electrode potential (EAPPLIED < ENERNSTIAN). The half reaction can be driven in the oxidizing (anodic) direction by applying a potential more positive than the equilibrium electrode potential (EAPPLIED > ENERNSTIAN).




