Cottrell Equation
Last Updated: 5/7/19 by Tim Paschkewitz
1Cottrell Equation
There are many resources that describe the detailed background, derivation, and applications of this equation.
 Bard, A. J.; Faulkner, L. A. Electrochemical Methods: Fundamentals and Applications, 2nd ed. Wiley-Interscience: New York, 2000.
Bard, A. J.; Faulkner, L. A. Electrochemical Methods: Fundamentals and Applications, 2nd ed. Wiley-Interscience: New York, 2000.
Kissinger, P.; Heineman, W. R. Laboratory Techniques in Electroanalytical Chemistry, 2nd ed. Marcel Dekker, Inc: New York, 1996.
Wang, J. Analytical Electrochemistry, 3rd ed. John Wiley & Sons, Inc.: Hoboken, NJ, 2006. Here, we present just a snippet to get you started.
Kissinger, P.; Heineman, W. R. Laboratory Techniques in Electroanalytical Chemistry, 2nd ed. Marcel Dekker, Inc: New York, 1996.
Wang, J. Analytical Electrochemistry, 3rd ed. John Wiley & Sons, Inc.: Hoboken, NJ, 2006. Here, we present just a snippet to get you started.
The Cottrell equation describes the current response, in time, as a function of a step in potential. For the general half-reaction,  and starting from the concentration profile with linear diffusion and assigning appropriate boundary conditions, the current-time response observed during an instantaneous potential step experiment is
and starting from the concentration profile with linear diffusion and assigning appropriate boundary conditions, the current-time response observed during an instantaneous potential step experiment is
where 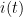 is current,
is current, 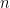 is the number of electrons transferred in the half reaction,
is the number of electrons transferred in the half reaction, 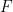 is Faraday's Constant (96,485 C/mol)
is Faraday's Constant (96,485 C/mol)
 Wikipedia - Faraday Constant
,
Wikipedia - Faraday Constant
, 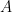 is the area of the electrode,
is the area of the electrode, 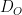 is the diffusion coefficient,
is the diffusion coefficient,  is the initial concentration, and
is the initial concentration, and 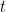 is time.
is time.
Written in linear form,
which has the form of 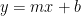 , therefore,
, therefore,
where
In this linear form, a plot of  will indicate deviations from linearity, which suggest that the electrochemical reaction is coupled to other processes such as kinetic limitations or molecular/chemical changes such as ligand association or dissociation or geometric rearrangements.
will indicate deviations from linearity, which suggest that the electrochemical reaction is coupled to other processes such as kinetic limitations or molecular/chemical changes such as ligand association or dissociation or geometric rearrangements.
2References
- Bard, A. J.; Faulkner, L. A. Electrochemical Methods: Fundamentals and Applications, 2nd ed. Wiley-Interscience: New York, 2000.
- Kissinger, P.; Heineman, W. R. Laboratory Techniques in Electroanalytical Chemistry, 2nd ed. Marcel Dekker, Inc: New York, 1996.
- Wang, J. Analytical Electrochemistry, 3rd ed. John Wiley & Sons, Inc.: Hoboken, NJ, 2006.



