
This article is part of the AfterMath Data Organizer Electrochemistry Guide
Interdependency of the Potentiostat and Electrochemical Cell
One of the key aspects of electrochemical measurements is that the potentiostat and the electrochemical cell are part of the same electrical circuit. If something is wrong with the cell, then the cell can make the potentiostat appear to have a problem. Or, if something is wrong with the potentiostat, it can cause an unwanted change to occur within the cell. Other kinds of laboratory instrumentation, from the lowly digital balance to more complex spectrophotometers and chromatographic detectors, are relatively immune to changes in the nature of the sample being analyzed and are less likely to cause unwanted changes in the composition of the sample. But the successful user of a potentiostat must always be aware of the significant interdependence between the potentiostat and the sample and plan experiments accordingly.
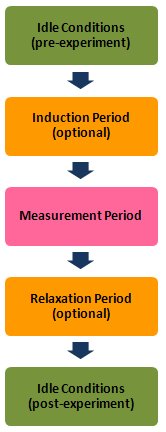
Part of this awareness is carefully managing the sequence of events before, during, and after an electroanalytical measurement. The initial conditions imposed upon an electrochemical cell (such as the working electrode potential) as well as the instrument settings (such as the current range) can have a direct bearing on the observed results of an electrochemical measurement. Similarly, the continuity of cell control (or lack thereof) after a measurement concludes can directly influence the composition of the portions of the electrochemical cell immediately adjacent to the electrode(s). Indeed, the surface properties of the electrodes themselves can be unwittingly altered by improper cell control.
Minimizing Cell Change for a Successful Electroanalytical Measurement
A strategy for a successful electroanalytical measurement often begins with devising a way to connect the electrodes to the potentiostat without any appreciable changes occurring within the cell. In the jargon of the electroanalytical chemist, this might be as simple as initially poising the working electrode at a potential where no Faradaic current flows. Or, in the jargon of the corrosion scientist, it might be wise to start an experiment with the corrosion sample at or near OCP (Open Circuit Potential, the potential at which there is no current at the working electrode).
The AfterMath software gives you control of the idle conditions which prevail while you are making electrode connections and before an experiment begins. These “pre-experiment” idle conditions can be adjusted at any time while the potentiostat is not doing an experiment. You can choose to hold the working electrode at a given potential under potentiostatic control or alternately, at a given current (usually zero amperes) under galvanostatic control. You may also choose to hold the electrode at OCP if you wish.
Click here for more information about adjusting pre-experiment idle conditions.
The Induction Period
As part of the experimental specifications for an electrochemical technique, AfterMath allows you to specify a period of time during which the cell is allowed to come into equilibrium with the initial signal levels applied to the working electrode(s). The period of time, called the induction period, is brief by default (usually less than 3 seconds); however, you may increase the duration of the induction period or you may skip the induction period if you wish.
Click here for more information about setting up the induction period.




Comments: