1Technique Overview
Cyclic Step Chronopotentiometry (CSCP) is a galvanostatic method also known as constant current (CC) multi-step in the battery field. It is related to galvanostatic bulk electrolysis and has several variants, including Chronopotentiometry (CP), Rotating Electrode (CP-RDE) and Rotating Cylinder (CP-RCE).
Cyclic Step Chronopotentiometry (CSCP) is a galvanostatic method in which the current at the working electrode is held at a constant level for a given period of time. The working electrode potential and current are recorded as a function of time. Up to four discrete steps can be defined for a CSCP experiment. These steps run sequentially until the experiment completes. Researchers employ this method to study chemical reaction mechanisms and kinetics. It is also frequently used to study batteries and electrodeposition. CSCP is typically performed in an unstirred electrochemical cell; although, some researchers employ rotating electrodes in conjunction with CSCP.
Typically, current is held constant between working and counter electrodes while potential is measured at the working electrode, relative to the reference electrode. Redox-active species diffuse to the working electrode surface to balance the applied current, until the diffusion-limited concentration of redox species reaches zero at the electrode surface, at which time potential changes to the redox potential of the next species, if present, in solution (which could be solvent). This process repeats at each step, or at each current level (positive or negative, increasing, decreasing, or both as inputted by the user) sequentially until the experiment completes the last step.
A single step CSCP experiment is exactly the same as a Chronopotentiometry (CP) experiment.
2Fundamental Equations
Numerous texts thoroughly explain electrochemical theory and should be consulted for a complete understanding of this method.
The theory of CSCP is the same as for CP.
Consider the general reaction
with formal potential
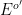
. As a constant current is applied to the working electrode,
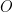
is reduced to
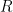
at the working electrode surface. As a result, the working electrode potential moves to values characteristic of the redox couple in a time-based Nernstian fashion. As the concentration of
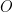
drops to zero at the electrode surface, the potential starts to rapidly increase to more negative values. The resulting
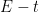
curve is much like a potentiometric titration with a transition time,

(analagous to titration equivalence point). The potential at one half

is
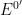
. The transition time is related to the concentration and diffusion coefficient of
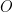
through the expression
where
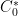
is the concentration of
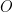
,
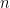
is the number of electrons,
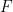
is
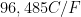
(Faraday's Constant),
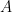
is the working electrode area,
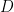
is the diffusion coefficient, and
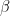
is the sweep rate.
For rapid electrode kinetics, the time-based Nernstian equation is
where
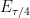
is equal to
A plot of
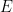
vs.

should give a straight line with slope of

for a reversible
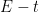
curve.
Additional and more complete background of this theory is provided in the Theory section of the Knowledgebase.
3Experimental Setup in AfterMath
To perform a cyclic step chronopotentiometry experiment in AfterMath, choose Cyclic Step Chronopotentiometry (CSCP) from the Experiments menu (see Figure 1).
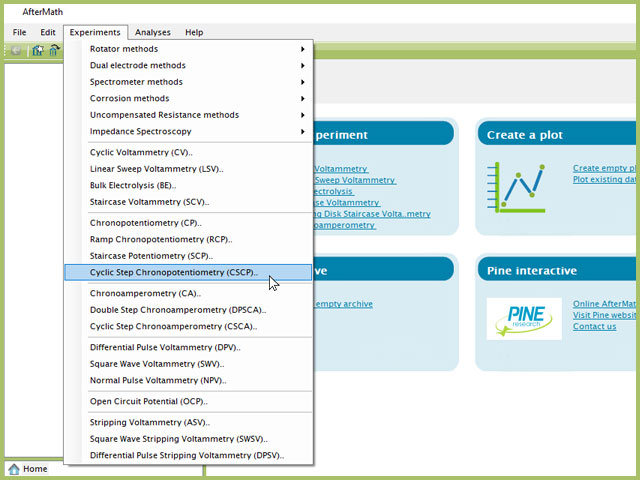
Figure 1. Cyclic Step Chronopotentiometry (CSCP) Experiment Menu Selection in AfterMath
Doing so creates an entry within the archive, called CSCP Parameters. In the right pane of the AfterMath application, several tabs will be shown (see Figure 2).
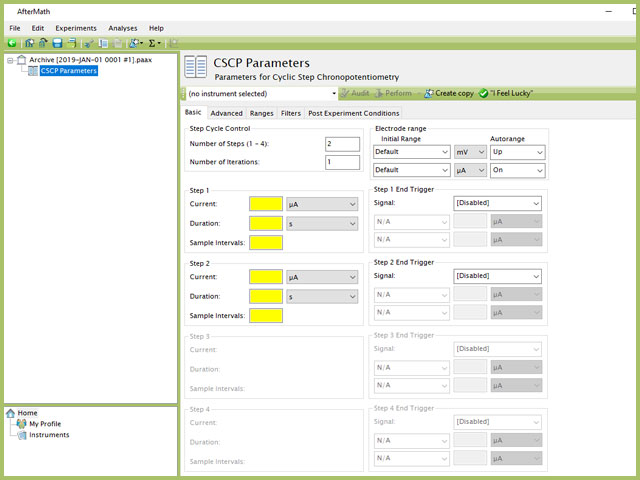
Figure 2. Cyclic Step Chronopotentiometry (CSCP) Experiment Parameters
As with most Aftermath methods, the experiment sequence is
Induction Period → Electrolysis Period (Step 1 - 4) → Relaxation Period → Post-Experiment Idle Conditions
The parameters for a CSCP experiment are fairly simple compared to other methods in AfterMath. Continue reading for detailed information about the fields on each unique tab.
3.1Basic Tab
TIP: Click the AutoFill button ("I Feel Lucky" prior to May 2019) on the top bar in AfterMath to automatically fill all required parameters with reasonable starting values. While the values provided may not be appropriate for your specific system, they are reasonable parameters with which to start your experiment, especially if you are new to the method.
The basic tab contains fields for the fundamental parameters necessary to perform a CSCP experiment. AfterMath shades fields with yellow when a required entry is blank and shades fields pink when the entry is invalid (see Figure 3).
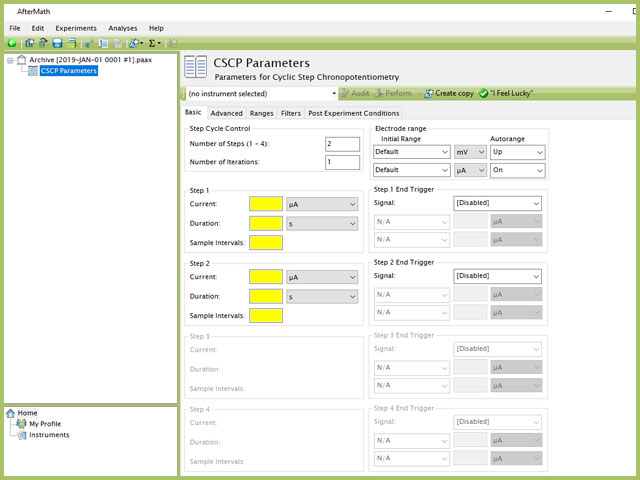
Figure 3. Cyclic Step Chronopotentiometry (CSCP) Basic Tab in AfterMath
During the induction period,
a set of initial conditions are applied to the electrochemical cell and the cell equilibrates at these conditions. Data are not collected during the induction period, nor are they shown on the plot during this period. Users will define induction period parameters on the Advanced Tab.
After the induction period, the current applied to the working electrode is stepped to the specified value for duration specified, which is called the electrolysis period, for each designated step in sequence. The galvanostatic circuit of the instrument maintains constant applied current while simultaneously measuring the potential at the working electrode relative to the reference electrode. During the electrolysis period, potential and current at the working electrode are recorded at regular intervals as specified on the Basic tab for each step.
The experiment concludes with a relaxation period.
During the relaxation period, a set of final conditions (specified on the Advanced tab) are applied to the electrochemical cell and the cell equilibrates at these conditions. Data are not collected during the induction period, nor are they shown on the plot during this period. Users will define relaxation period parameters on the Advanced Tab.
After the induction period, the current applied to the working electrode is stepped to the specified value for the duration of the step, which is called the electrolysis period (on CSCP, the electrolysis periods are found within individual groups, labeled Step 1, Step 2, Step 3, and Step 4). For CSCP, users can specify up to four steps. Each step requires three inputs: Current, Duration, and Sample Intervals. Users cannot adjust the order of steps, rather AfterMath will perform each step in sequential order (i.e., 1, 2, 3, 4).
NOTE: The "Number of Steps" default in AfterMath is 2. Up to four individual steps can be entered, based on the value entered in this initial field. As shown in Figure 3, when 2 steps are selected in the Step Cycle Control group, only Step 1 and Step 2 are available for input, while Step 3 and Step 4 are grayed out. Changing the number of steps to 3 and 4 will enable the corresponding step input fields lower in the form.
The galvanostatic circuit of the instrument maintains constant applied current while simultaneously measuring the potential at the working electrode. During the electrolysis period, potential and current at the working electrode are recorded at regular intervals as specified on the Basic tab for the first potential step (Step 1) followed by each successive step, as indicated by "Number of Steps (1 - 4) in the Step Cycle Control group. There is no pause between the two steps and potential is nearly instantaneously stepped from the one electrolysis potential to the next.
Sampling intervals input box requests a number of data points (intervals) for the given step, spread across the step duration during electrolysis. With duration, a sampling rate can be defined as,
Users should enter a number of intervals that their analysis requires. Commonly, CSCA experiments are sampled at a lower rate (1 sample/s). Not all sampling rates are allowed due to differences in hardware (WaveNow series vs. WaveDriver series, for example).
NOTE: Not all sampling rates are possible. When users enter a number of intervals that is not allowed, AfterMath will prompt the user with an "Interval too short" error. Change the number of intervals and try again. In general, integer values at moderate rates are most often possible.
A plot of the typical experiment sequence, containing labels of the fields on the Basic tab, helps to illustrate the sequence of events in a CSCP experiment (see Table 1 and Figure 4).
Field labels in AfterMath will eventually include symbols, such as those used in the waveform plot below (see Figure 4). Consult the cross-reference table (see Table 1) to match field names with symbols.
| Group Name |
Field Name |
Symbol |
| Step Cycle Control |
Number of Steps |
 |
| Step Cycle Control |
Number of Iterations |
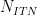 |
| Step n (N = 1, 2, 3, 4) |
Current |
 |
| Step n (N = 1, 2, 3, 4) |
Duration |
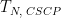 |
| Step n (N = 1, 2, 3, 4) |
Sample Intervals |
 |
Table 1. Basic Tab Group Names, Field Names, and Symbols
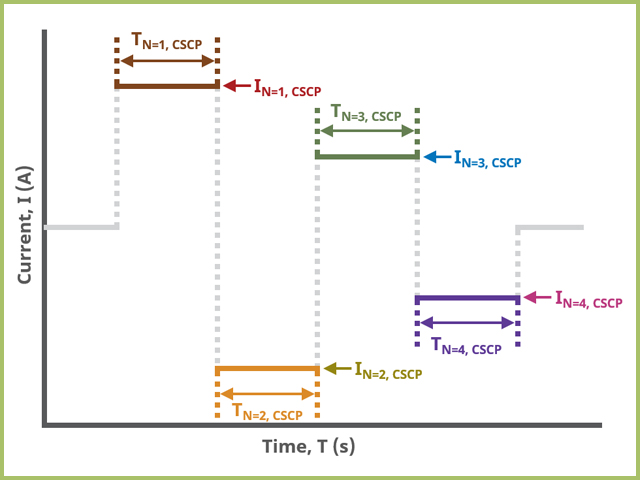
Figure 4. CSCP Field Diagram for Basic Tab in AfterMath
The Basic tab for CSCP also contains Step N End Trigger fields, which can be set on a per-step basis. In a CSCP experiment, the researcher might want to have AfterMath monitor the response (input) and then stop the experiment a specific current, potential, and/or charge. This value is called a trigger and is set in the Step N End Trigger group on the Basic tab (see Figure 5). Each step can have its own end trigger. For CSCP, the signals for the trigger can be potential or current. A common example of using this trigger is in the charging of a battery. A researcher would charge the battery by performing a CSCP experiment (also called a CC or constant current) with one trigger set to its upper potential limit (e.g., 100% SOC). Once this potential limit is reached, the CSCP experiment continues to the next step (if present), repeating until the final step completes or the end trigger for the final step terminates the experiment. More information about end triggers is available on the Knowledgebase.
At the end of the relaxation period (settings on the Advanced tab), the post-experiment idle conditions are applied to the cell, and the instrument returns to the idle state. The default plot generated from the data is measured potential vs. time, called a chronoamperogram.
3.2Advanced Tab
The CSCP Advanced tab contains groups for Induction Period, Relaxation Period, Experiment End Trigger, and iR Compensation (see Figure 5).
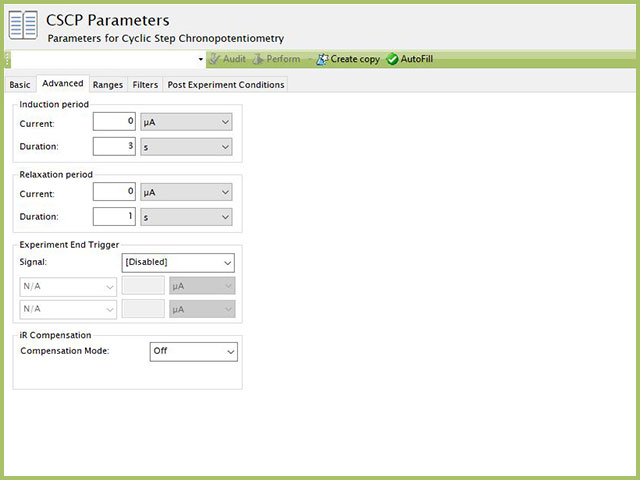
Figure 5. Cyclic Step Chronopotentiometry (CSCP) Advanced Tab in AfterMath
Induction Period is the first step in a CSCP experiment if the Duration is >0 s. During the induction period, the specified current is applied to the cell for the specified duration. During this period, data are not collected. The Induction Period is believed to "calm" the cell prior to intentional perturbation. More on Induction Period is found within the knowledgebase.
Relaxation Period is the last step in a CSCP experiment if the Duration is >0 s. During the relaxation period, the specified current is applied to the cell for the specified duration. During this period, data are not collected. The Relaxation Period is believed to "calm" the cell after intentional perturbation. More on Relaxation Period is found within the knowledgebase.
To visualize the induction and relaxation periods, consider the general sequence of events during a CSCP experiment (see Figure 6 and Table 2).
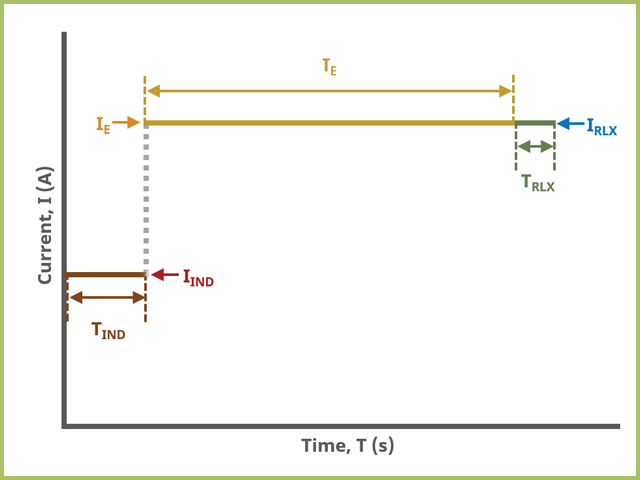
Figure 6. Cyclic Step Chronopotentiometry (CSCP) experiment timing in AfterMath
Field labels in AfterMath will eventually include symbols, such as those used in the timing plot above (see Figure 6). Consult the cross-reference table (see Table 2) to match field names with symbols.
| Group Name |
Field Name |
Symbol |
| Induction Period |
Current |
 |
| Induction Period |
Duration |
 |
| Relaxation Period |
Current |
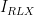 |
| Relaxation Period |
Duration |
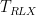 |
Table 2. Advanced Tab Group Names, Field Names, and Symbols
Experiment End Trigger is different than the Step N End Triggers on the Basic tab because the value of this Experiment End Trigger overrides any individual Step N Triggers (on the Basic tab). When Experiment End Trigger condition arises the experiment entirely terminates, including any unfinished steps and/or iterations. The Step N End Triggers, when reached, end that step, but the experiment is allowed to proceed to any next steps (or iterations).
Lastly, the iR Compensation group allows users to adjust the cell feedback to accommodate a known resistive drop between working and reference electrodes. Not all potentiostats from Pine Research support iR compensation. The WaveDriver series support iR compensation by positive feedback and current interrupt. The WaveDriver 100
and WaveDriver 200
support EIS-based iR compensation. The WaveNow
series (including the WaveNano
and WaveNowXV
) and the CBP bipotentiostat do not support iR compensation of any type. More information about iR compensation, including understanding how it works and how to determine the resistance, consult the knowledgebase article on the topic.
3.3Ranges, Filters, and Post Experiment Conditions Tab
In nearly all cases, the groups of fields on the Ranges tab are already present on the Basic tab. The Ranges tab shows an Electrode Range group and depending on the experiment shows either, or both, current and potential ranges and the ability to select an autorange function. The fields on this tab are linked to the same fields on the Basic tab (for most experiments). Changing the values on either the Ranges tab or on the Basic tab changes the other set. In other words, the values selected for these fields will always be the same on the Ranges tab and on the Basic tab. More on ranges is found within the knowledgebase,
as is for autorange.
The Filters tab provides access to potentiostat hardware filters, including stability, excitation, current response, and potential response filters. Pine Research recommends that users contact us
for help in making changes to hardware filters. Advanced users may have an easier time changing the automatic settings on this tab. Filter settings fields are shown for WK1 (working electrode #1) as well as for WK2 (working electrode #2) regardless of the potentiostat connected to AfterMath.
By default, the potentiostat disconnects from the electrochemical cell at the end of an experiment. There are other options available for what these post-experiment conditions can be and are controlled by setting options on the Post Experiment Conditions tab.
4Applications from Literature
In the 1960's, Herman and Bard described the theoretical basis for single component
and multicomponent systems and stepwise reactions
using CSCP. The results were used to calculate the half-wave potential of each scan of a single component system, which was then compared to lead ion reduction for verification.
Molina and González et. al have published many articles in which CSCP proved a useful method for many applications, including the study of CEC, CE, EC, and catalytic mechanisms
and the study of charge transfer reactions,
.
5References
-
Bard, A. J.; Faulkner, L. A. Electrochemical Methods: Fundamentals and Applications, 2nd ed. Wiley-Interscience: New York, 2000.
-
Kissinger, P.; Heineman, W. R. Laboratory Techniques in Electroanalytical Chemistry, 2nd ed. Marcel Dekker, Inc: New York, 1996.
-
Wang, J. Analytical Electrochemistry, 3rd ed. John Wiley & Sons, Inc.: Hoboken, NJ, 2006.
-
Herman, H. B. ; Bard, A. J. Cyclic Chronopotentiometry. Diffusion Controlled Electrode Reaction of a Single Component System.. Anal. Chem., 1963, 35(9), 1121-1125.
-
Herman, H. B. ; Bard, A. J. Cyclic Chronopotentiometry. Multicomponent Systems and Stepwise Reactions.. Anal. Chem., 1964, 36(6), 971-975.
-
Molina, A. ; González, J. ; López‐Tenés, M. Application of the superposition principle to the study of CEC, CE, EC and catalytic mechanisms in cyclic chronopotentiometry. Part III. J. Math. Chem., 1998, 23(3), 277-296.
-
Molina, A. ; González, J. ; Serna, C. ; Camacho, L. Application of the superposition principle to the study of a charge transfer reaction in cyclic chronopotentiometry. Part II. J. Math. Chem., 1996, 20(1), 169-181.






 WaveDriver 100 EIS Potentiostat Basic Bundle
and WaveDriver 200
WaveDriver 100 EIS Potentiostat Basic Bundle
and WaveDriver 200
 WaveDriver 200 EIS Bipotentiostat Basic Bundle
support EIS-based iR compensation. The WaveNow
WaveDriver 200 EIS Bipotentiostat Basic Bundle
support EIS-based iR compensation. The WaveNow
 Classic WaveNow Potentiostat
series (including the WaveNano
Classic WaveNow Potentiostat
series (including the WaveNano
 Classic WaveNano Potentiostat
and WaveNowXV
Classic WaveNano Potentiostat
and WaveNowXV
 WaveNowXV Potentiostat/Galvanostat Basic Bundle
) and the CBP bipotentiostat do not support iR compensation of any type. More information about iR compensation, including understanding how it works and how to determine the resistance, consult the knowledgebase article on the topic.
WaveNowXV Potentiostat/Galvanostat Basic Bundle
) and the CBP bipotentiostat do not support iR compensation of any type. More information about iR compensation, including understanding how it works and how to determine the resistance, consult the knowledgebase article on the topic.



