This article is part of the AfterMath Data Organizer Electrochemistry Guide.
Detailed Description
Like most of the other electrochemical techniques offered by the AfterMath software, this experiment begins with an induction period. During the induction period, a set of initial conditions is applied to the electrochemical cell and the cell is allowed to equilibrate to these conditions. The default initial condition involves holding the working electrode potential at the initial potential for a brief period of time (i.e., 3 seconds). After the induction period, the potential of the working electrode is stepped to a specified potential for a period of time. After the step has finished, the experiment concludes with a relaxation period. The default condition during the relaxation period involves holding the working electrode potential at the initial potential for an additional brief period of time (i.e., 1 seconds). At the end of the relaxation period, the post experiment idle conditions are applied to the cell and the instrument returns to the idle state.
Current is plotted as a function of time, resulting in a chronoamperogram. You may also choose to do some post experiment processing in order to generate a Cottrell plot.
Parameter Setup
The parameters for this method are arranged on various tabs on the setup panel. The most commonly used parameters are on the Basic tab. Additional tabs for Ranges and post experiment idle conditions are common to all of the electrochemical techniques supported by the AfterMath software. Finally, a Post Experiment Processing tab deals with manipulating the data automatically when the experiment is finished.
Basic Tab
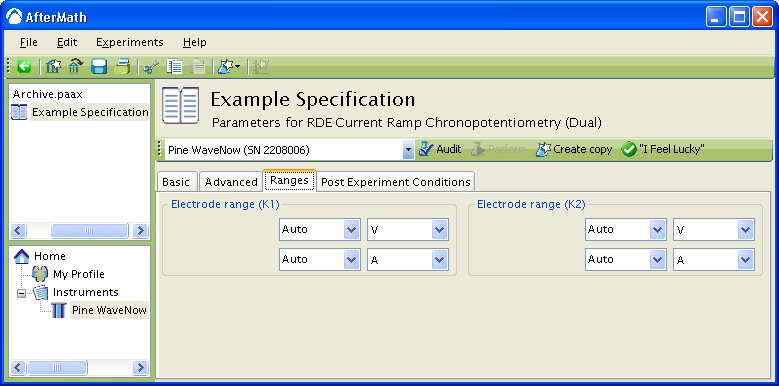
You can click on the “I Feel Lucky” button (located at the top of the setup) to fill in all the parameters with typical default values (see Figure 1). You will no doubt need to change the Potential and Hold time in the Forward step period box to values which are appropriate for the electrochemical system being studied. You may also want to change the Number of intervals in the Sampling Control box.
Figure 1: Basic Chronoamperometry Setup
The Electrode Range on the Basic tab is used to specify the expected range of current. If the choice of electrode range is too small, actual current may go off scale and be truncated. If the electrode range is too large, the chronoamperogram may have a noisy, choppy, or quantized appearance.
Some Pine potentiostats (such as the WaveNow and WaveNano portable USB potentiostats) have current autoranging capability. To take advantage of this feature, set the electrode range parameter to “Auto”. This allows the potentiostat to choose the current range “on-the-fly” while the chonoamperogram is being acquired.
The waveform that is applied to the electrode is a simple pulse to the Potential listed in the Forward step period box. Note that the actual waveform that is measured (see Figure 2, red trace) fluctuates slightly compared to the applied potential (see Figure 2, black trace).
Figure 2: Waveform for CA. Black trace = applied potential, Red trace = measured potential.
Ranges Tab
AfterMath has the ability to automatically select the appropriate ranges for voltage and current during an experiment. However, you can also choose to enter the voltage and current ranges for an experiment. Please see the separate discussions on autoranging and the Ranges Tab for more information.
Post Experiment Conditions Tab
After the Relaxation Period, the Post Experiment Conditions are applied to the cell. Typically, the cell is disconnected but you may also specify the conditions applied to the cell. Please see the separate discussion on post experiment conditions for more information.
Post Experiment Processing Tab
Typical Results
The results for a solution of ferrocene in
are shown in Figure 4.
Figure 4: Chronoamperogram of a ferrocene solution using a potential = and a
Pt WE.
If you selected to automatically generate Cottrell plots, the plots are under the other plots folder in the Archive navigation panel. Choosing Cottrell current displays a plot of (see Figure 5A). Choosing Cottrell charge displays a plot of
(see Figure 5B). Note that for the Cottrell Current plot, the level portion in the plot is actually the time prior to the current spike shown in Figure 4. That is, earlier time points are to the right in a Cottrell Current Plot. This is not the case for a Cottrell Charge plot because integrating the current with respect to time gives charge. The endpoint in a Cottrell Charge plot is the total amount of charge passed during the experiment. Monitoring the charge passed during an experiment is a variant of chronoamperometry called Chronocoulometry. See the Applications section for an example of Chronocoulometry.
Figure 5 : Post experiment processing plots. A – Cottrell Current , B – Cottrell Charge
. Conditions as listed in Figure 4.
The Cottrell current plot is a useful diagnostic tool to examine that your species of interest is freely diffusion in solution. Upon applying the potential step, the current initially spikes, then begins trail off. As will be explained in more detail in the Theory section, the current during the trailing off period is a diffusion-limited current dictated by the Cottrell equation. Examining the Cottrell Current plot in this region reveals that the current is linear with respect to (see black ellipse in Figure 6).
Figure 6: Highlight of Diffusion-limited Current in Cottrell Current Plot.
The addition of a Baseline tool to the diffusion limited current region allows for calculation of the diffusion coefficient for the species of interest if the concentration and electrode area are known (see Figure 7). First you will have to delete the region of the Cottrell Plot where current is not diffusion limited. Use the Point Selection Tool to delete the unwanted points of the Cottrell Plot. Next, add a baseline tool by right clicking on the leftover point and select Add Tool » Baseline. Manipulate the control points to provide an adequate fit. AfterMath automatically provides the slope and intercept for the Baseline tool.
Figure 7: Addition of Baseline Tool in Diffusion-limited Current Region.
The slope of the line in the plot is given by the equation
where is the number of electrons,
is Faraday’s Constant
,
is the electrode area
,
is the diffusion coefficient
, and
is the concentration
. In the example above,
is calculated to be
.
Theory
The theory section is split into two segments. The first segment deals with Chronoamperometry (CA) and the second section deals with Chronocoulometry (CC). Chronoamperometry leads to Chronocoulometry natually since charge is obtained by integrating current with respect to time.
Chronoamperometry
The following is a basic description of the theory behind Chronoamperometry. Please see the literature1 for a more detailed description of the technique. Consider a reaction with a formal potential
. In general, the potential step applied to the working electrode should be sufficiently more negative than
such that reduction of
to
is complete at the surface of the electrode (i.e. surface concentration of
at the electrode surface is
). When this occurs, the current is diffusion-limited, much like the current that flows in CV after the potential of the electrode sweeps past
.
When the current is diffusion-limited in CA, the current-time response is described by the Cottrell2 equation
where
is the number of electrons,
is Faraday’s Constant
,
is the electrode area
,
is the diffusion coefficient
, and
is the concentration
. As described in the Typical Results section, plotting
gives a slope that contains the diffusion coefficient,
.
Chronocoulometry
The following is a brief description of chronocoulometry. Please see the literature1 for a more detailed description of the technique. Chronocoulometry is advantages in some ways over chronoamperometry. First is that the signal grows with time, meaning that the later portion of the experiment are least distorted by the nonideal potential rise, giving better signal-to-noise ratios. Second, integrating smooths random noise making the data cleaner. Third, contributions from double-layer charging and reactions of adsorbed species can be separating from those of freely diffusion species. The cumulative charge passed during the experiment is given by the equation
where the parameters are as described in the above section. Typically, a plot of has a non-zero y-intercept. This intercept is related to double-layer charging and reduction of adsorbed
. The overall equation describing the charge is then given by
where is the charge due to double-layer charging and
is the charge due to reduction of adsorbed
.
and
are not easily separated and usually require additional experiments such as double potential step chronoamperometry. However, approximations for
can be made by performing the same potential step in a solution of only electrolyte. This is only an approximation because the true double-layer charging will be influenced by adsorbed
.
The Application section in the next tab contains examples of both Chronoamperometry and Chronocoulometry.
Application
In the first example, Tennyson et al.3 used chronoampometry to determine the diffusion coefficients (D) and number of electrons (n) for a series of indirectly connected bimetallic complexes. In this instance, the researchers used microelectrodes to generate steady-state currents. Plotting of the function
allows for the calculation of without knowledge of
or
. Though the experiments are relatively simple and straightforward, the researchers then used these values for more complicated electrochemical experiments that enabled them to determine the degree of electronic coupling between the two metallic centers. Understanding the degree of electronic coupling could allow for facilitation to otherwise inaccessible catalysts and materials.
The next example uses chronoamperometry to measure extremely small diffusion coefficients of redox polyether hybrid cobalt bipyridine molten salts. Crooker and Murray4 performed chronoamperometry on a series of undiluted molten salts and were able to obtain diffusion coefficients as low as . The researchers went on to obtain heterogeneous rate constants
using CV and were able to show that the reaction remains quasireversible despite small
rates because
values are so low.
The next example uses chronoamperometry to directly measure rate constants. Smalley et al.5 examined a series of monolayers terminated with either a ferrocene or ruthenium redox moiety. For monolayers containing more than 11 methylene units, chronoamperometry was used to determine the heterogeneous rate constants . The researchers also determined preexponential factors and found them to be much lower that expected based aqueous solvent dynamics.
The fourth example uses chronocoulometry. Wolfe et al.6 prepared a series of ferrocenated hexanethiolate protected Au nanoparticles. Normallly, TGA would be used to calculate the number of thiolates per nanoparticle. However, in this case, TGA was inconclusive due to a slow loss of mass over the applied temperature range. The researchers instead monitored the charge pass over time for solution of nanoparticles with known concentrations. Knowing the total charge passed and the particle concentration allowed them to determine the number of ligands per nanoparticle. This is a good example of how electrochemistry can be used in place of a more traditional characterization technique.
The final example also uses chronocoulometry, but rather than using it for quantification purposes, the researchers use it to evoke a change in the system. Zhang et al.7 use chronocoulometry with predefined endpoints to produce Ag nanoparticles, confined on a electrode surface, with varying shapes and sizes. By varying the amount of charge passed the researchers were able to show that triangular nanoparticles oxidize first on the bottom edge, followed by triangular tips, followed by out-of-plane height. Since the researchers also coupled spectroscopy to their electrochemical experiments they were also able to show how the SPR band changes with shape evolution. This is a nice example of how electrochemistry can be used to evoke a change in a system rather than simply to monitor changes or determine physical parameters of the system.
References
- Faulkner, L. R.; Bard, A. J. Basic Potential Step Methods, Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New Jersey, 2000; 156-225.
- Cottrell, F. G. Z. Physik, Chem., 42, 1902, 385.
- Tennyson, A. G.; Khramov, D. M.; Varnado, C. D. Jr.; Creswell, P. T.; Kamplain, J. W.; Lynch V. M.; Bielawski, C. W.; Organometallics, 2009, 28, pp 5142–5147.
- Crooker, J. C.; Murray, R. W. Anal. Chem., 2000, 72, pp 3245–3252.
- Smalley, J. F.; Finklea, H. O.; Chidsey, C. E. D.; Linford, M. R.; Creager, S. E.; Ferraris, J. P.; Chalfant, K.; Zawodzinsk, T.; Feldberg, S. W.; Newton, M. D. J. Am. Chem. Soc., 2003, 125, pp 2004–2013.
- Wolfe, R. L.; Balasubramanian, R.; Tracy, J. B.; Murray, R. W. Langmuir, 2007, 23 (4), pp 2247–2254.
- Zhang, X.; Hicks, E. M.; Zhao, J.; Schatz, G. C.; Van Duyne, R. P. Nano Lett., 2005, 5, pp 1503–1507