Electrochemical Analysis of Acetaminophen in Pain Relief Medication
Last Updated: 11/1/19 by Alex Peroff
Download as PDF1Abstract
Potter, D. W.; Miller, D. W.; Hinson, J. A. Identification of Acetaminophen Polymerization Products Catalyzed by Horseradish Peroxidase. J. Biol. Chem., 1985, 260(22), 12174-12180. Designed for use in an instructional laboratory setting, this procedure describes the use of cyclic voltammetry to verify the concentration of acetaminophen (4-acetamidophenol) indicated on the suspension label. Advantages of the approach used here include the use of inexpensive and disposable screen-printed carbon electrodes and the use of an inexpensive consumer-grade supporting electrolyte solution that is widely available on the mass market. The only reagent grade chemical required for this procedure is a small amount of pure 4-acetamidophenol.
2Introduction

Figure 1. Experimental Apparatus and Chemicals
3Background
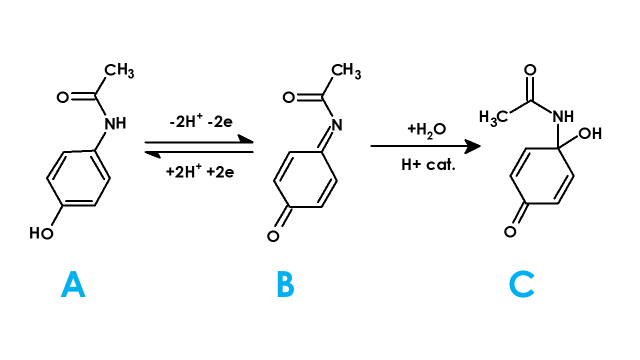
Figure 2. Electrochemical Reaction of Acetaminophen
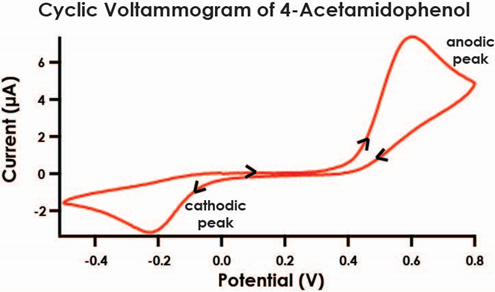
Figure 3. Quasi-Reversible Electrochemical Process of 4-Acetamidophenol
4Experimental
4.1Instrumentation
- WaveNow Potentiostat/Galvanostat System
 WaveNowXV Potentiostat Bundles
connected to a PC with AfterMath Data Organizer installed
WaveNowXV Potentiostat Bundles
connected to a PC with AfterMath Data Organizer installed
 AfterMath Downloads
AfterMath Downloads
- Compact Voltammetry Cell (Pine Research part number AKSPEKIT)
 Screen-Printed Electrode Cell Starter Kit
Screen-Printed Electrode Cell Starter Kit
- Screen-Printed Carbon Electrodes with Spacers (Pine Research part number RRPE1001C-100)
 Carbon Screen-Printed Electrodes
Carbon Screen-Printed Electrodes
4.2Chemicals
- Contact Lens saline buffer. The major ingredients of this buffer (pH~7.3 ) are boric acid, sodium borate, and sodium or potassium chloride
- 4-Acetamidophenol CH2CONHC6H4OH (151.2 g/mol)
- Children’s Acetaminophen Medication, oral suspension
4.3Solution Preparation
4.3.110.0 mM Acetaminophen Stock Solution
4.3.2Children’s Acetaminophen Medication Stock Solution
4.3.3Acetaminophen Calibration Standards
| Acetaminophen Stock Solution (mL) |
Buffer Solution (mL) |
Concentration (mM) |
| 0.00 | 10.0 | 0.00 |
| 0.20 | 9.80 | 0.20 |
| 0.40 | 9.60 | 0.40 |
| 0.60 | 9.40 | 0.60 |
| 0.80 | 9.20 | 0.80 |
| 1.00 | 9.00 | 1.00 |
4.3.4Diluted Medication Test Solution (unknown)
5Procedure
5.1Background Voltammetry
5.1.1Set Up the Potentiostat and Cell
- Turn on the WaveNow potentiostat and connect it to a personal computer using a USB cable. Use the AfterMath software application to control the potentiostat.
- Connect the cell cable to the potentiostat using the end terminated with an HD-15 connector. The other end of the cable should be a mini-USB connector.
- Obtain a screen-printed electrode (SPE) (see Figure 4). These patterned electrodes have a working electrode in the center which has a 2.0 mm diameter. The working electrode area is defined by a blue masking material which coats the underlying carbon ink. Compute the surface area of the working electrode (in cm2) and make a note of your result in your lab notebook.
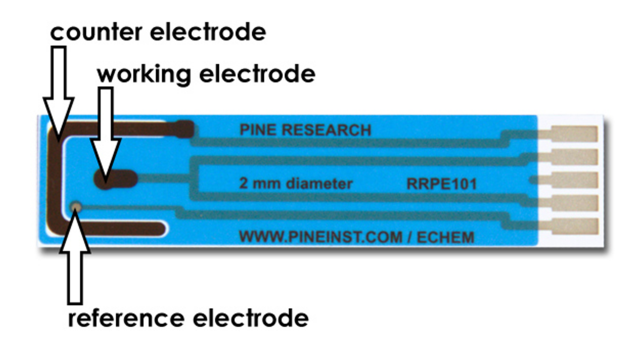
Figure 4. Working, Counter, and Reference Electrodes on a Carbon SPE
- The electrode pattern also includes a counter electrode (large, black U-shaped electrode) and a silver/silver chloride reference electrode (a small grey dot located slightly off-center).
- Fasten the compact voltammetry cell cap to the glass vial that contains the blank standard solution. Carefully mount one carbon SPE onto the hand-grip. Put one to two spacers on the back side of the SPE, and use the spacers to secure proper electrical contact between the SPE and the pins in the blue electrode connector (see Figure 5).

Figure 5. Compact Voltammetry Cell Components: (a) USB-Style Cell Cable; (b) Cell Grip Mount; (c) Screen-Printed Electrode (SPE); (d) Scintillation Vial; and (e) Cap
- Refer to Figure 5 for the following instructions: insert the cell grip mount (b), loaded with the SPE (c), into the cap (e),which is screwed onto the scintillation vial (d), and connect the USB-style cell cable (a) to the USB-style port on the left side of the cell grip mount.
- The completely assembled cell is shown in Figure 6.

Figure 6. Assembled Compact Voltammetry Cell, with USB Cable Connected to the Left
5.1.2Performing the Background Scan with Experimental Parameters
- Place Contact Saline Buffer solution into the electrochemical cell.
- In AfterMath, select the Cyclic Voltammetry option from the Experiments menu, and then enter the experimental parameters shown in Figure 7. Note that these parameters are suggested starting values for obtaining a background voltammogram. It may be necessary to make some minor adjustments to obtain a satisfactory voltammogram. In particular, it may be necessary to adjust the current range to optimize the appearance of the voltammogram.
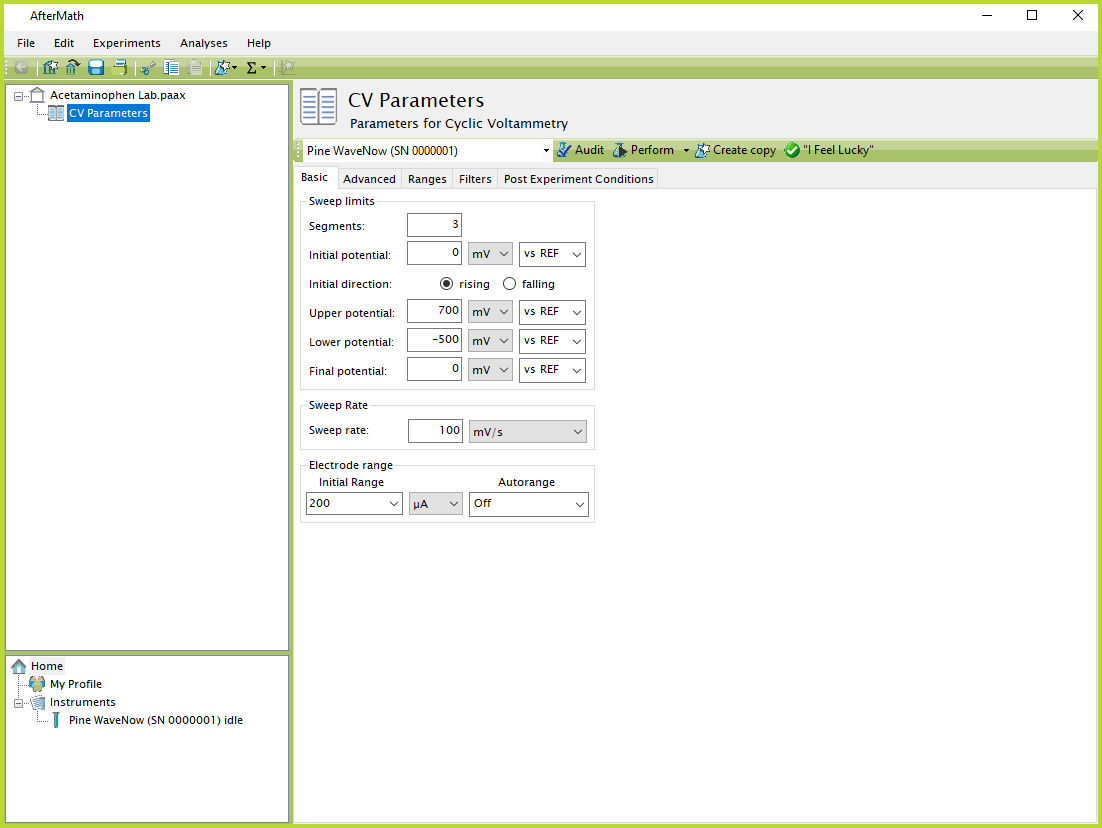
Figure 7. AfterMath CV Experimental Parameters
- Click the "Perform" button to initiate the experiment. You may wish to repeat the experiment using different current ranges until an optimum range is found. Alternately, you can use the "On" setting for current Autorange and allow the software to select the optimal range automatically.
- The background cyclic voltammogram should be relatively featureless (other than random sub-microampere noise) and exhibit no significant peaks. The overall background current should be less than 500 nA. If significant peaks are apparent, then the buffer, the glassware, and/or the electrode surface are likely contaminated. Consult with your instructor if excessive or unusual background current is observed.
- After acquiring a satisfactory background voltammogram, save the data archive to the disk so that you do not lose your data. Give the archive a filename which is meaningful to you (such as “Acetaminophen Lab”). Save the archive file to the disk frequently as you continue to acquire more results.
- After each measurement using an SPE, rinse it with saline solution, and dry it either by gently blotting with a piece of paper towel or by blowing a stream of dry nitrogen over the electrode surface.
- Note that each time you use a new SPE, you should repeat this procedure and obtain a new background cyclic voltammogram. In this way, you can verify that the surface of the SPE has not been inadvertently contaminated by mishandling or touching the surface of the electrode.
5.1.3Voltammograms of Calibration Standards
- Create a new folder in your data archive called “Standard Solutions” by right-clicking on the data archive (left hand panel), and choosing the “New > Folder” menu option. Use the mouse to drag the background voltammogram and the CV parameters into this new folder.
- As before, obtain cyclic voltammograms for each of the five remaining standard solutions that you have prepared. Set the electrode current range to 200 μA for all CV measurements, and use the same sweep rate (100 mV/s) for all solutions. When a solution in a vial is not in use, be sure to keep the vial securely capped. Each voltammogram should exhibit a prominent anodic peak near 0.5 V and a smaller cathodic peak near -0.2 V (see Figure 3).
- After each voltammogram is obtained, be sure to rename it with a name that helps you to remember the corresponding solution concentration. Move each voltammogram into the “Standard Solutions” folder that you created above.
- Obtain a cyclic voltammogram for the Diluted Medication Test Solution and save your results.
5.1.4Voltammogram of Acetaminophen Medication Solution (Unknown)
- Create a new folder in your data archive called “Unknown Solutions” by right-clicking on the data archive (left hand panel), and choosing the “New > Folder” menu option. Use the mouse to drag the background voltammogram and the CV parameters into this new folder.
- As before, obtain a cyclic voltammogram for the diluted acetaminophen medication solution. Set the electrode current range to 200 μA for all CV measurements, and use the same sweep rate (100 mV/s) for this solution. When a solution in a vial is not in use, be sure to keep the vial securely capped. The voltammogram should exhibit a prominent anodic peak near 0.5 V and a smaller cathodic peak near -0.2 V as you observed for the calibration standards (see Figure 3).
- After each the voltammogram is obtained, be sure to rename it to indicate it is the data obtained for the diluted medication of unknown concentration. Move the voltammogram into the “Unknown Solutions” folder that you created above.
6Data Analysis
6.1Peak Height Analysis
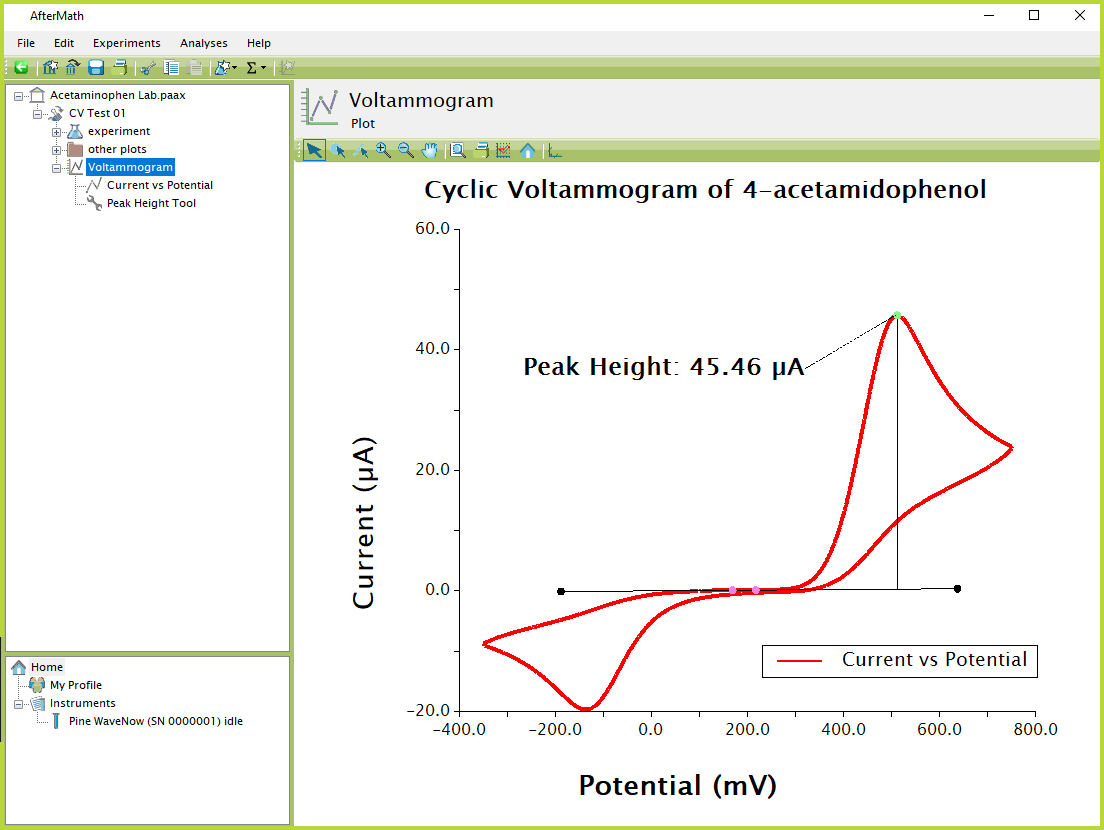
Figure 8. AfterMath Acetaminophen CV
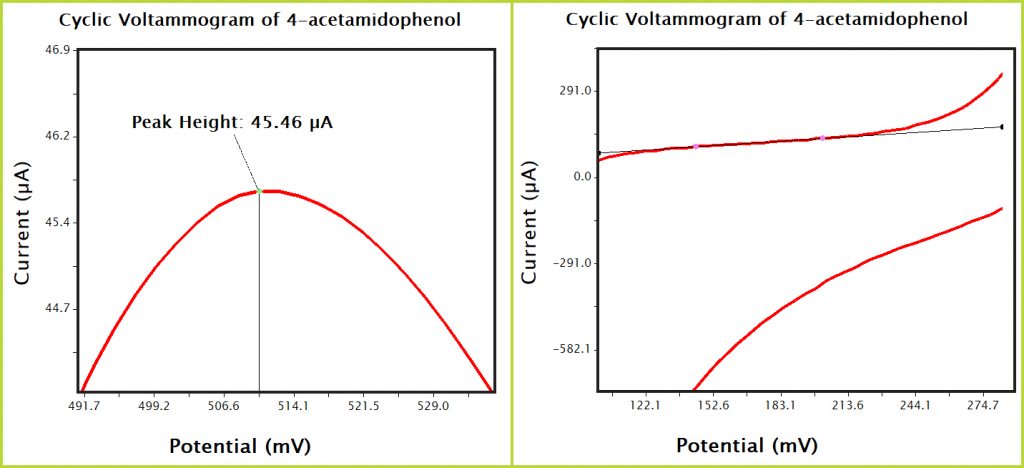
Figure 9. AfterMath Acetaminophen CV Zoomed
6.2Calibration Curve
- Using the anodic peak height data, prepare the calibration curve of peak current versus acetaminophen concentration. This can be done using a piece of graph paper, or more preferably, a spreadsheet program such as Microsoft Excel.
- Use the linear least squares method to fit a straight line to the data on the calibration curve. Calculate the slope and the intercept of best straight line that fits the data, and write down the equation that correlates the peak current with the acetaminophen concentration.
6.3Concentration in Children's Tylenol® Suspension
7Report Questions
- List at least two reasons, outside of your control, that could explain why your result differs from that listed on the manufacturer’s label.
- How would your results have been affected if you had failed to measure the anodic peak current against the proper background baseline?
- Examine the peak height shown in Figure 8. Given that the oxidation of acetaminophen involves the removal of two electrons from the molecule, how many molecules per second must be oxidized in order to produce the observed peak current?
- From your cyclic voltammogram data analysis, compare your determination of acetaminophen concentration with the value given on the label of the commercial medication. Assuming the manufacturer’s label gives the “correct” concentration, what was the percent error in your result?
8References
- Van Benschoten, J. J. ; Lewis, J. Y. ; Heineman, W. R. ; Roston, D. A. ; Kissinger, P. T. Cyclic Voltammetry Experiment. J. Chem. Educ., 1983, 60(9), 772.
- Potter, D. W.; Miller, D. W.; Hinson, J. A. Identification of Acetaminophen Polymerization Products Catalyzed by Horseradish Peroxidase. J. Biol. Chem., 1985, 260(22), 12174-12180.



